Abstract
Objective
To evaluate the effects of n-hexane insoluble fraction (HIF) of Ficus septica leaves in combination with doxorubicin on cytotoxicity, cell cycle and apoptosis induction of breast cancer T47D cell lines.
Methods
The in vitro drugs-stimulated cytotoxic effects were determined using MTT assay. Analysis of cell cycle distribution was performed using flowcytometer and the data was analyzed using ModFit LT 3.0 program. Apoptosis assay was carried out by double staining method using ethydium bromide-acridin orange. The expression of cleaved-poly (ADP-ribose) polymerase (PARP) on T47D cell lines was identified using immunocytochemistry.
Results
The combination exhibited higher inhibitory effect on cell growth than the single treatment of doxorubicin in T47D cells. In addition, combination of doxorubicin and HIF increased the incidence of cells undergoing apoptosis. HIF could improve doxorubicin cytotoxic effect by changing the accumulation of cell cycle phase from G2/M to G1 phase. The combination also exhibited upregulation of cleaved-PARP in T47D cells.
Conclusions
Based on this results, HIF is potential to be developed as co-chemotherapeutic agent for breast cancer by inducing apoptosis and cell cycle arrest. However, the molecular mechanism need to be explored further.
Keywords: Ficus septica Burm. F., Cancer, T47D cells, Cytotoxic
1. Introduction
The use of medicinal plants in the community has been increasing for several decades[1]. Some of them are already used in the formal health services. A medicinal plant before launched in community or used in the formal health services should be evaluated for its efficacy, safety and acceptability. The medicinal plants are evaluated for their quality based on chemical and pharmaceutical assays, preclinical assays and clinical trials in order to estimate the benefits and risks of their use[2]. Pharmacological quality of medicinal plant is assessed in in vitro and in vivo studies. In line with this, screening of Indonesian medicinal plants have been widely done. One of the potential medicinal plants is Ficus septica Burm. F (F. septica).
Reportedly, the ethanolic extract of F. septica showed a cytotoxic effect on breast cancer T47D cell lines with IC50 value of 13 µg/mL[3]. The extract at 4.88 µg/mL also showed an optimum synergistic effect in combination with doxorubicin (3.75 nmol)[4]. In addition, the extract induced apoptosis and downregulated the expression of Bcl-2 protein in breast cancer cells MCF-7[5]. In an in vitro study, the ethanolic extract (750 mg/kg body weight) induced apoptosis through p53-independent pathway in liver cancer of 7,12-dimethylbenz[a]nthracene-induced rat[6].
In previous study, the ethanolic extract of F. septica Burm. F. leaves was gradually fractionated using n-hexane and ethyl acetate yielding hexane soluble fraction, n-hexane insoluble fraction (HIF), ethyl acetate soluble fraction and ethyl acetate insoluble fraction[7]. Among them, HIF showed the most potent cytotoxic effect on T47D breast cancer cell lines with IC50 value of 9 µg/mL. In the study, we subsequently investigated the effect of the HIF in combination with doxorubicin on cytotoxicity, cell cycle and apoptosis induction of breast cancer T47D cell lines. This study is useful for further development of herbal medicine in vivo as well as clinic as co-chemotherapy agents for cancer treatment.
2. Materials and methods
2.1. Materials
F. septica leaves were collected from area around Sumber Arum Moyudan, Yogyakarta, Indonesia and was identified by a botanist at Department of Pharmaceutical Biology, Universitas Gadjah Mada, and the voucher specimen was deposited in herbarium of the department. Doxorubicin (Ebewe) was obtained from P.T. Ferron Par Pharmaceutical (Cikarang, Indonesia). DMSO was obtained from Sigma Aldrich Chemie GmBH Germany, and was used for in vitro experiment by diluting desired concentration. The final DMSO concentration was made with a concentration of less than 0.1%. Other materials were [3-(4,5-dimetilthiazol-2-yl)-2.5-diphenyl tetrazolium bromide] (MTT) (Sigma Chemical, St. Loius, MO, USA), H2O2 (Lab Vision Plus) and chromogen 3, 3-diaminobenzidin (Novo Castra).
2.2. Preparation of hexane insoluble fraction (HIF)
Briefly, dried ground powder of fresh leaves of F. septica was extracted using 70% ethanol. The filtrate was collected, and then evaporated under reduced pressure to give viscous ethanolic extract. The extract was added with 100 mL aquadest to yield liquid form of ethanolic extract. The extract was fractionated with n-hexane yielding n-hexane soluble fraction and insoluble fraction of n-hexane. The insoluble fraction was then concentrated by rotary vacuum evaporator to obtain viscous extract. The fraction was dried using freeze drying to eliminate the existence of the remaining traces of water.
2.3. Cell culture
In the study, T47D cells (a human breast carcinoma) were obtained from Prof. Masashi Kawaichi (Nara Institute Sciences and Technology, Japan). Vero cells were collected from Cancer Chemoprevention Research Center, Faculty of Pharmacy UGM. T47D cells were grown in Dulbecco's modified eagles medium, while Vero cells were grown in M199 medium containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 1% penicillin-1% streptomycin (Gibco), and 0.5% fungizon (Gibco) in a flask in a humidified atmosphere (5% CO2) at 37 ˚C.
2.4. Cell viability assay
T47D and Vero cell viabilities were assessed using MTT colorimetric assay. The cells were cultured in 96-well plates (Becton Dickinson Co., NJ, USA). Each well contained 5×103 cells. The culture cells were incubated in a humidified incubator at 37 ˚C in an atmosphere of 5% CO2 and 95% air for 24 h. After 24 h incubation, the medium was discharged and treated by HIF in combination with doxorobicin. After incubation for 24 h, the cells were incubated with 0.5 mg/mL of MTT for 4 h at 37 ˚C. Viable cells react with MTT to produce purple formazan crystals. After 4 h, the stopper 10% SDS (Sigma Co., St. Louis, MO) in 0.01 mol HCl (Merck) was added to dissolve the formazan crystal. The cells were then incubated for 24 h at room temperature and protected from light. After incubation, the cells were shaken, and cells absorbance was measured by ELISA reader at λ 595 nm. The experimental data were absorbance of each well, and then converted to percentage of viable cells[8].
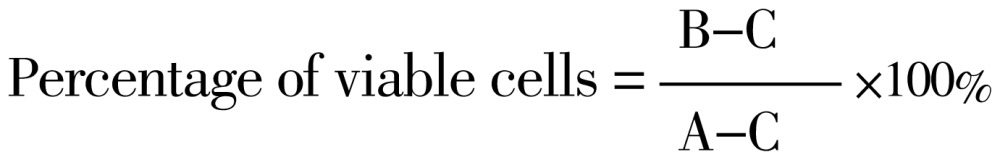 |
Where A, B and C are absorbances of control group, treatment group and medium (vehicle), respectively.
Selectivity index was calculated using equation below[9].
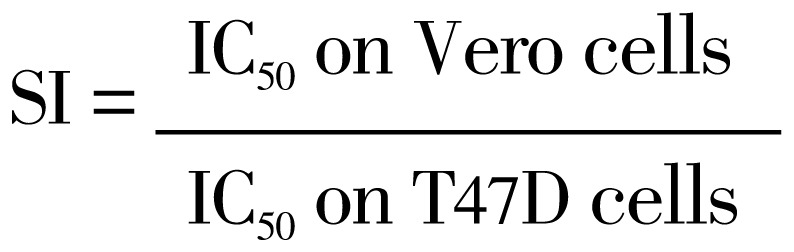 |
2.5. Apoptosis assay
T47D cells were transfered on coverslips placed in 24-well plate to obtain the density of 5×104 cells/well, and then incubated for 24 h (50-60 confluent). After that, the cells were treated by HIF, doxorubicin or their combination, and then incubated for 15 h. The coverslips were immediately moved to object glass. Etidium bromide-acridine orange (Sigma-Aldrich, St. Louis, MO, USA) were added to the cells and then allowed to stand for 5 min. The cells were immediately observed by fluorescence microscope (Zeiss MC80). Green fluorescence cells indicate viable cells, and orange fluorescence cells indicate apoptosis cells.
2.6. Flowcytometry assay
T47D cells (5×105 cells/well) were seeded into 6-well plate and incubated for 24 h. After that, the cells were treated by HIF, doxorubicin or their combination, and then incubated for 24 h. Both floating and adherent cells were collected using 0.025% tripsin, and transferred into a 1.5 mL tube. The cells were washed twice with cold PBS and centrifuged. The supernatant was discharged, while the pellet was collected and fixed gently in cold 70% ethanol in PBS at -20 ˚C for 1 h. The fixed cells were then washed twice with cold PBS and resuspended in PBS containing PI (40 µg/mL), RNAse (100 µg/mL) and TritonX-100 at 37 ˚C for 30 min. The samples were then analysed using FACScan flowcytometer. Based on DNA contents, percentage of cells in each stage of cell cycle (G1, S and G2/M phases) were calculated using ModFit Lt. 3.0.s.
2.7. Immunocytochemistry
T47D cells were seeded at 5×105 cells/well on coverslips in 24-well plate until 80% confluent (24 h incubation). The medium was then replaced by fresh medium containing HIF, doxorubicin or their combination, and then incubated in a humidified incubator at 37 ˚C in an atmosphere of 5% CO2 and 95% air for 24 h. After incubation, the cells were washed with PBS and then fixed with cold methanol for 10 min at -4 ˚C. Afterward, the cells were washed with PBS and blocked in hydrogen peroxide blocking solution for 10 min at room temperature. The cells were incubated with primary antibody of cleaved-poly (ADP-ribose) polymerase (cleaved-PARP) for 1 h at room temperature. The cells were washed three time with PBS, then incubated with secondary antibody for 10 min. After washing with PBS, the cells were incubated in 3,3 diaminobenzidin solution for 10 min and then washed with aquadest. After that, the cells were counterstained with Mayer-Haematoxylin for 3 min. After incubation, the coverslips were taken and washed with aquadest, and then immersed with xylol and alcohol. The expression of protein cleaved-PARP was determined using an light microscope (Nikon, Japan) and photographed using an digital camera (Canon, Japan). Positive and negative expression of protein cleaved-PARP were represented by dark brown and purple colour in nucleus, respectively.
2.8. Statistical analysis
All data were expressed as mean±SEM. One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test were used for statistical analyses. P-values less than 0.05 were considered significant.
3. Results
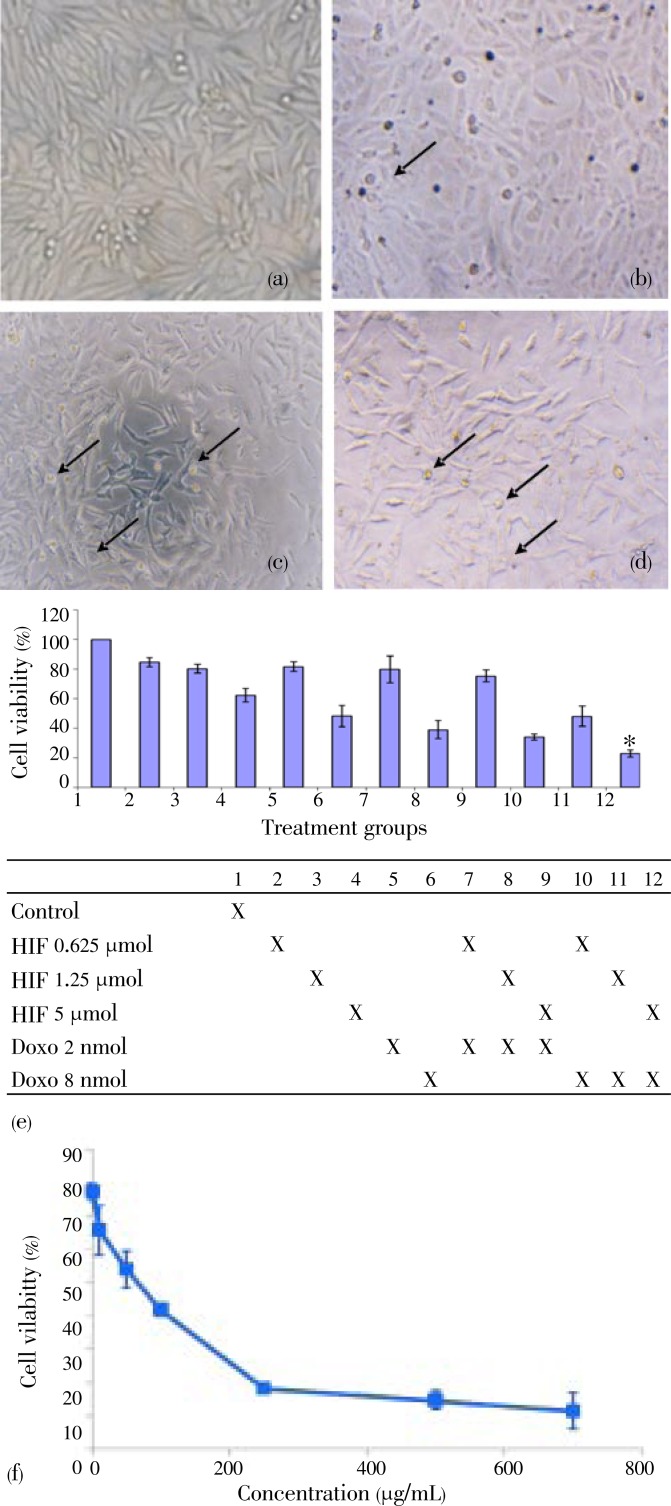
Morphological change of the cells were more extensive after treatment with combination of HIF and doxorubicin than that with single treatments (Figure 1a-1d). All treatments (HIF, doxorubicin and their combinations) succeeded to inhibit cells growth in concentration-dependent manner (Figure 1e). Combination of doxorucin with HIF showed higher inhibitory effects on cell growth than that of the single treatments of doxorubicin in T47D cells (Figure 1e). The results indicated that HIF enhanced cytotoxic effect of doxorubicin in T47D breast cancer cell lines. These effects are supposed to be related to apoptotic induction and cell cycle modulation.
Figure 1. Combination effects of doxorubicin and n-HIF of F. septica on cells morphology after incubation with vehicle.
a: control; b: HIF 5 µg/mL; c: doxorubicin 8 nmol; d: combination of doxorubicin 8 nmol and HIF 5 µg/mL; e: cell viability (%) of T47D cells using MTT method; f: cell viability profile of HIF on Vero cells. Data were expressed as (mean±SD). *Combination was considered significant comparing with doxorubicin single treatment (8 nmol) (P<0.01).
To measure the selectivity of HIF, we performed cell viability assay on Vero cells. Single treatment of HIF showed cytotoxic effect on Vero cells with IC50 30 µg/mL (Figure 1f). We compared IC50 of HIF on Vero to T47D cells to get selectivity index (SI)[9]. SI of HIF>3 is supposed to be selective to T47D cancer cells. The results showed that HIF was selective to T47D cells rather than Vero cells.
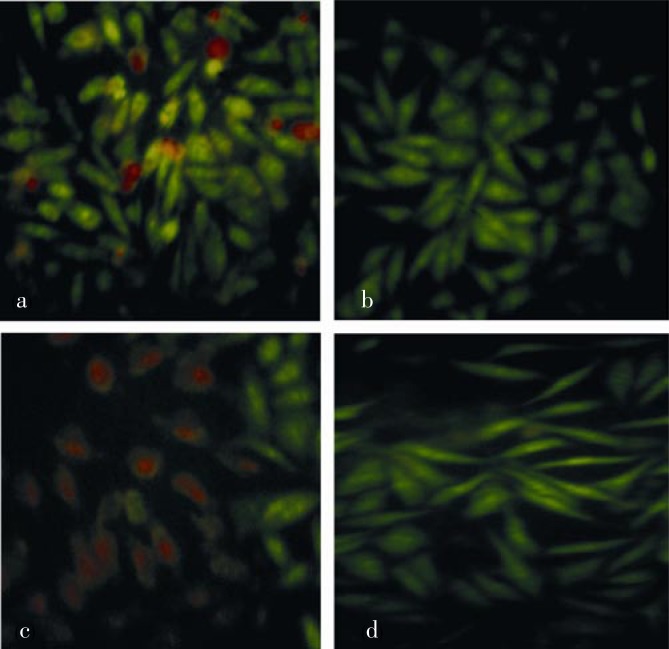
Evaluation of apoptosis induction was performed using double-staining method with ethidium bromide-acrydine orange staining. As shown in Figure 2a, control cells showed green fluorescence indicating no cell death. Viable cells only absorbed acridine orange. Viable cell showed a good cell integrity so that ethidium bromide could not penetrate into the cells. Orange fluorescence indicates apoptotic cells due to loss of cell membrane permeability and the cells form apoptotic bodies. Single treatment of HIF did not induce apoptosis (Figure 2b), while single treatment of doxorubicin showed weak apoptosis induction (Figure 2c). More intensive orange fluorescence was shown in the cells with treatment of combination of doxorubicin 8 nmol and HIF 5 µg/mL (Figure 2d).
Figure 2. Effects of doxorubicin, HIF and their combination on apoptosis induction in T47D cells after 24 h incubation.
a: control cells; b: HIF 5 µg/mL; c: doxorubicin 8 nmol; d: combination of doxorubicin 8 nmol and HIF 5 µg/mL. Viable and death cell were represented by green and orange fluorescence, respectively. Combination of HIF and doxorubicin increased apoptosis induction of doxorubicin single treatment on T47D cells.
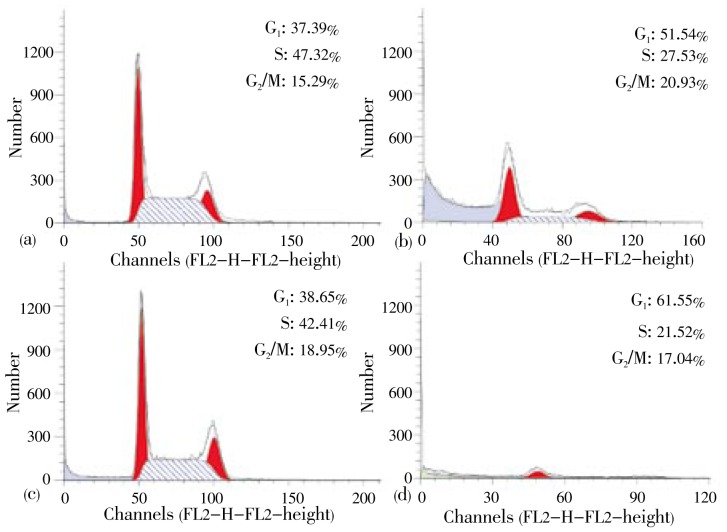
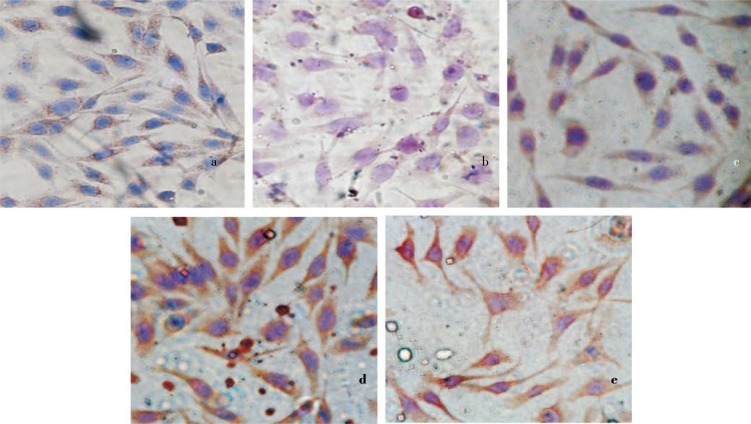
To confirm that treatment of HIF in combination with doxorubicin increased cell death by modulating cell cycle, we evaluated cell cycle profile using flowcytometry method. In the study, treatment of HIF alone increased G1 accumulation. Whereas, single treatment of doxorubicin dominantly caused cell accumulation at G2/M phase on T47D cells (Figure 3). Furthermore, combination of doxorubicin and HIF exhibited higher G1 accumulation than their single treatments. The combination also caused decrease of cell population compared to their single treatment. These facts indicate that HIF could improve doxorubicin cytotoxic effect by changing the inhibition of cell cycle phase G2/M to phase G1. PARP is main protein found in nucleus that plays a role in cellular reactions mainly DNA repair and programmed cell death. Inhibition on PARP through PARP cleavage causes death only in tumor or cancer cells. In this case, DNA repair by homologous recombination was inhibited. In the study, effects of HIF, doxorubicin or their combination on c-PARP expressions were investigated imunohistochemically. Expression of c-PARP protein was positively characterized by brown-stained nuclei in the cells (Figure 4a). In contrast, there was no brown-stained nuclei found in the control cells without antibody (Figure 4b). In untreated cells (negative control), low intensity of brown area was observed in the cells indicating low expression of c-PARP protein. A single treatment of HIF (Figure 4c) and doxorubicin (Figure 4d) did not increase the expression of c-PARP in T47D cells compared to control cells. Combination treatment of doxorubicin and HIF exhibited more intensive stimulatory effect on c-PARP expression in T47D cells than this of single treatment of doxorubicin (Figure 4e). It showed that combinational treatment upregulated c-PARP on T47D cells.
Figure 3. Cell cycle analysis using flowcytometry.
T47D cells were treated by doxorubicin, HIF and their combination for 24 h and stained using propidium iodide. (a) control cells; (b) HIF 5 µg/mL; (c) doxorubicin 8 nmol; (d) combination of HIF 5 µg/mL and doxorubicin 8 nmol. Combination of HIF and doxorubicin induced G1 cell arrest and decreased T47D cell population.
Figure 4. Expression of c-PARP on T47D cells using immunohistochemistry.
a: control cells with antibody; b: control cells without antibody; c: HIF 5 µg/mL; d: doxorubicin 8 nmol; e: and combination of doxorubicin 8 nmol and HIF 5 µg/mL. Expression of c-PARP protein is positive characterized by brown-stained cytoplasm in the cells. Combination of HIF and doxorubicin increased upregulation of c-PARP compared to their single treatment.
4. Discussion
Phytochemical study, active compounds in the extract were compounds of the flavonoid and alkaloid[10]. The extract was then fractionated gradually yielding several fractions. One of them showed potent cytotoxic effect was n-HIF with IC50 value of 9.4 µg/mL[7]. We investigated the effect of n-HIF on cytotoxicity, cell death induction, and cell cycle of T47D cells with single treatment and in combination with doxorubicin. We also checked selctivity of HIF on Vero cells. HIF showed selectivity on T47D cells compared to Vero cells with SI value[9]. Doxorubicin is a chemotherapeutic agent showing strong cytotoxic effect on T47D cell lines with IC50 value of 15 nmol[11]. T47D cells could be resistant to doxorubicin due to p53 mutation[12],[13]. Therefore, to prevent the tendency of resistence on T47D cells, combination of low concentration of doxorubicin with HIF is needed. In the study, combination of HIF and doxorubicin exhibited synergism effect on T47D cells. HIF enhanced the cytotoxic effect of doxorubicin on T47D cells in comparison to single treatment of either HIF or doxorubicin. The synergistic effect is suggested to be related to apoptotic induction and cell cycle modulation. In apoptosis study, in line with cytotoxic study the combination of doxorubicin and HIF increased the incidence of cells undergoing apoptosis in comparison with single treatment of doxorubicin or HIF on T47D cells. Apoptosis is programmed cell death characterized by changes on cell morphology, membrane blebbing and chromatine[14]. Activation of executioner protein of apoptosis e.g. as caspase 3 and caspase 7 induced cleavage of distinct cellular protein, mainly PARP[15]. In the study, low intensity of brown area in control cells associated with low expression of cleaved-PARP protein indicates that PARP did not undergo any cleavage. PARP cleavage has a crusial role in apoptosis of cancer cells. Combination of HIF and doxorubicin increased the expression of cleaved-PARP in T47D cells. It indicates that induction of apoptosis due to the treatment might be related to PARP cleavage. PARP is cell signaling enzyme involved in DNA damage repairment and in poly ADP-ribosylation of DNA binding proteins that cause modification of nuclear proteins[16],[17]. DNA repair is important for the survival of normal and cancer cells[18]. Inhibition on PARP through PARP cleavage can prevent the repair of DNA damage, and contribute in apoptosis process[19]. In the cell cycle analysis, a single treatment of chemotherapeutic agent doxorubicin dominantly caused cell accumulation at G2/M phase on T47D cells. In the other side, treatment of HIF alone caused G1 accumulation in T47D cells. Combination treatment of doxorubicin and HIF exhibited higher G1 accumulation in comparison with single treatments of doxorubicin or HIF. Combinational treatment also decreased cell population. Based on the results, HIF could improve doxorubicin cytotoxic effect by changing the inhibition of cell cycle phase G2/M to phase G1.
Our present study showed that combination of HIF and doxorubicin increased apoptosis induction via G1-arrest. Some proteins regulate G1 arrest such as p21 and p27. Upregulation of p21 on breast cancer cells is p53 independent[20]. Treatment of DNA damaging agent on human cancer cells induce cell cycle arrest mediated by p21 and followed by apoptosis after cleave of p21 mediated by caspase3[21]. Cells with p53 mutant are more potent to induce apoptosis via p21 than cells with p53 wild type[22]. We proposed that increasing apoptosis of doxorubicin by combination of HIF and doxorubicin occured via p21-mediated apoptosis, but this mechanism needs to be explored further. The use of doxorubicin together with HIF is expected to increase the activity and reduce the side effects of doxorubicin. However, the molecular mechanism of apoptotic induction and cell cycle by this combination need to be explored with more details. Based on the results, we concluded that combination of n-HIF of F. septica leaves and doxorubicin synergically increases the cytotoxic effect of doxorubicin through apoptosis (cell death induction), increase of cleaved-PARP expression and cell cycle arrest. The fraction is potential to be developed as co-chemotherapeutic agent for doxorubicin in breast cancer therapy.
Acknowledgments
We gratefully thank DP2M DIKTI (Directorate of Higher Education) Ministry of Education, Indonesia through “Hibah Bersaing” Research Grant 2011 for financial support in the study.
Comments
Background
Doxorubicin, one of the popular chemotherapeutic agents, frequently induces resistance and shows side effects, such as heart failure. To reduce these adverse effects, extracts of medicinal plants may be used in a combinatorial treatment with doxorubicin. Screening of Indonesian medicinal plants has revealed F. septica as potential source of co-chemotherapeutic agents.
Research frontiers
Studies are conducted to clarify the cytotoxic effects of the hexane insoluble fraction of F. septica leaves in the combination treatment with doxorubicin on T47D breast cancer cells in terms of the interference with cell cycle and apoptosis processes.
Related reports
A series of researches were conducted by the authors' group to identify active compounds in different fractions of the F. septic extract. This paper presents a novel finding that the hexan insoluble fraction contains compounds that increase the cytotoxic effects of doxorubicin.
Innovations and breakthroughs
The hexan insoluble fraction of F. septica extract potentiates the cytotoxic effects of doxorubicin through apoptotic induction and G1 phase cell cycle arrest.
Applications
The results of this work suggest that hexane insoluble fraction of F. septica leave extract can be used clinically as co-chemotherapeutic agent for doxorubicin on breast cancer patients. The usage of the fraction may reduce the dosage of doxorubicin and prevent development of adverse effects of this drug.
Peer review
This is a good beneficial research which explores an Indonesian medicinal plant as a source of co-chemotherapeutic agents for doxorubicin in the combination treatment of breast cancer. This work reveals that usage of hexane insoluble fraction of F. septica leaves have potential to be developed as co-chemotherapy for doxorubicin against breast cancer.
Footnotes
Foundation Project: Supported by DP2M DIKTI (Directorate of Higher Education) Ministry of Education, Indonesia through “Hibah Bersaing” Research Grant 2011).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mikail CN, Hearney E, Nemesure B. Increasing physician awareness of the common uses and contraindications of herbal medicines: utility of a case-based tutorial for residents. J Altern Complement Med. 2003;9(4):571–576. doi: 10.1089/107555303322284866. [DOI] [PubMed] [Google Scholar]
- 2.Miroddi M, Calapai F, Calapai G. Potential beneficial effects of garlic in oncohematology. Mini Rev Med Chem. 2011;11(6):461–472. doi: 10.2174/138955711795843293. [DOI] [PubMed] [Google Scholar]
- 3.Nurcahya BM. Efek antiproliferatif ekstrak etanolik daun awar-awar (Ficus septica Burm. f.) terhadap sel kanker payudara T47D, skripsi. Yogyakarta: Fakultas Farmasi UGM; 2007. [Google Scholar]
- 4.Pratama RH, Ikhtiarsyah YG, Fitriasari A, Anindyajati, Ikawati M, Meiyanto E. Awar-awar leaves ehanolic extract sinergistically enhances cytotoxic of doxorubicin on T47D breast cancer cells. Ind J Pharm Sci. 2011;9(1):67–71. [Google Scholar]
- 5.Sekti DA, Mubarok MF, Armandani I, Junedy S, Meiyanto E. Awar-awar (Ficus septica Burm. F.) leaves ethanolic extract induced apoptosis of MCF-7 cells by downregulation of Bcl-2. J Trad Med. 2010;15(3):100–104. [Google Scholar]
- 6.Septhea DB, Anindyajati, Darma A, Nurzijah I, Nugroho AE. Ficus septica burm. F. leaves ethanolic extract induces apoptosis in 7,12-dimethylbenz[a]nthracene-induced rat liver cancer quantitatively. Indo J Cancer Chemoprev. 2010;2(2):242–248. [Google Scholar]
- 7.Nugroho AE, Ikawati M, Hermawan A, Putri DDP, Meiyanto E. Cytotoxic effect of ethanolic extract fractions of Indonesia plant Ficus septica Burm. F. on human breast cancer T47D cell lines. Int J Phytomed. 2011;3:216–226. [Google Scholar]
- 8.Mosmann T. Rapid colorimetric assay for cellular growth and survival: aplication to proliferation and citotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 9.Prayong P, Barusrux S, Weerapreeyakul N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia. 2008;79:598–601. doi: 10.1016/j.fitote.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Wu PL, Rao KV, Su CH, Kuoh CS, Wu TS. Phenanthroindolizidine alkaloids and their cytotoxicity from the leaves of Ficus septica. Heterocycles. 2002;57:2401–2408. [Google Scholar]
- 11.Junedi S, Susidarti RA, Meiyanto E. Naringenin meningkatkan efek sitotoksik doxorubicin pada sel kanker payudara T47D melalui induksi apoptosis. Ind J Pharm Sci. 2010;8(2):85–90. [Google Scholar]
- 12.Di Leo A, Tanner M, Desmed C, Paesman M, Cardoso F, Durbecq V, et al. et al. p-53 gene mutations as a predictive marker in a population of advanced breast cancer patients randomly treated with doxorubicin or docetaxel in the context of a phase III clinical trial. Ann Oncol. 2007;18:997–1003. doi: 10.1093/annonc/mdm075. [DOI] [PubMed] [Google Scholar]
- 13.Vayssade M, Haddada H, Faridoni-Laurens L, Tourpin S, Valent A, Benard J, et al. et al. p73 functionally replaces p53 in adriamycin-treated, p53-deficient breast cancer cells. Int J Cancer. 2005;116(6):860–869. doi: 10.1002/ijc.21033. [DOI] [PubMed] [Google Scholar]
- 14.Rudin CM, Thompson CB. Regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 15.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin. 2005;37(11):719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 16.Sodhi RK, Singh N, Jaggi AS. Poly (ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol. 2010;53(3–4):77–87. doi: 10.1016/j.vph.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Peralta-Leal A, Rodríguez MI, Oliver FJ. Poly(ADP-ribose)polymerase-1 (PARP-1) in carcinogenesis: potential role of PARP inhibitors in cancer treatment. Clin Transl Oncol. 2008;10(6):318–323. doi: 10.1007/s12094-008-0207-8. [DOI] [PubMed] [Google Scholar]
- 18.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, et al. et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274(33):22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 19.Rios J, Puhalla S. PARP inhibitors in breast cancer: BRCA and beyond. Oncology. 2011;25(11):1014–1025. [PubMed] [Google Scholar]
- 20.Pellikainen MJ, Pekola TT, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. p21WAF1 expression in invasive breast cancer and its association with p53, AP-2, cell proliferation, and prognosis. J Clin Pathol. 2003;56:214–220. doi: 10.1136/jcp.56.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Fujita N, Tsuruo T. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene. 1999;18:1131–1138. doi: 10.1038/sj.onc.1202426. [DOI] [PubMed] [Google Scholar]
- 22.Kaneuchi M, Yamashita T, Shindoh M, Segawa K, Takahashi S, Furuta I, et al. et al. Induction of apoptosis by the p53-273L (Arg3Leu) mutant in HSC3 cells without transactivation of p21Waf1/Cip1/Sdi1 and bax. Mol Carcinog. 1999;26:44–52. [PubMed] [Google Scholar]