Table 4.
SAR of oxime and methyloxime analogs
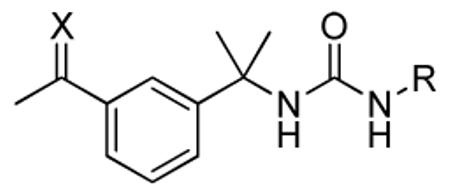
| ID | X | R | IC50 (nM) | |
|---|---|---|---|---|
| (−) BSA | (+) BSA | |||
| 7a | N-OH | 3-CF3,4-Cl-Ph | 1.0 ± 0.1 | 4 ± 2 |
| 7b | N-OH | 3-NO2,4-Cl-Ph | 0.66 ± 0.08 | 2.0 ± 0.4 |
| 7c | N-OH | 3-CONH2, 4-Cl-Ph |
5 ± 1 | 6 ± 2 |
| 7d | N-OH | 2-Naphthyl | 1.0 ± 0.2 | 2.3 ± 0.6 |
| 7e | N-OH | 7-Quinolyl | 0.9 ± 0.2 | 1.2 ± 0.3 |
| 8a | N-OMe | 3-CF3, 4-Cl-Ph | 5 ± 1 | 18 ± 3 |
| 8b | N-OMe | 3-CONH2, 4-Cl-Ph |
5 ± 2 | 5 ± 1 |
| 8c | N-OMe | 2-Naphthyl | 1.6 ± 0.1 | 13 ± 4 |
| 9 | N-O(CH2)2NH2 | 3-NO2,4-Cl-Ph | 20 ± 3.4 | 24 ± 17 |
