Abstract
Differentiating embryonic stem cells (ESCs) can form ovarian follicle-like structures in vitro, consisting of an oocyte-like cell surrounded by somatic cells capable of steroidogenesis. Using a dual-fluorescence reporter system in which mouse ESCs express green fluorescent protein (GFP) under the control of a germ cell–specific Pou5f1 gene promoter and red fluorescent protein (Discosoma sp red [DsRed]) driven by the granulosa cell–specific Forkhead box L2 (Foxl2) gene promoter, we first confirmed in vitro formation of follicle-like structures containing GFP-positive cells surrounded by DsRed-positive cells. Isolated DsRed-positive cells specified from ECSs exhibited a gene expression profile consistent with granulosa cells, as revealed by the detection of messenger RNAs (mRNAs) for Foxl2, follistatin (Fst), anti-Müllerian hormone (Amh), and follicle-stimulating hormone receptor (Fshr) as well as by production of both progesterone and estradiol. In addition, treatment of isolated DsRed-expressing cells with follicle-stimulating hormone (FSH) significantly increased estradiol production over basal levels, confirming the presence of functional FSH receptors in these cells. Last, ESC-derived DsRed-positive cells injected into neonatal mouse ovaries became incorporated within the granulosa cell layer of immature follicles. These studies demonstrate that Foxl2-expressing ovarian somatic cells derived in vitro from differentiating ESCs express granulosa cell markers, actively associate with germ cells in vitro, synthesize steroids, respond to FSH, and participate in folliculogenesis in vivo.
Keywords: granulosa cells, Foxl2, embryonic stem cell, oocyte, follicle, ovary
Introduction
The first report that differentiating mouse embryonic stem cells (ESCs) can spontaneously form oocyte-like cells in vitro was published nearly a decade ago.1 A very recent article has now demonstrated that fully functional eggs can be generated from oocytes initially derived from mouse ESCs or induced pluripotent stem cells (iPSCs).2 Specification of primordial germ cell (PGC)-like cells (PGCLCs) from differentiating mouse ESCs or iPSCs follows a precise time frame in vitro, with PGCLCs formed between 3 and 6 days into the differentiation program closely resembling endogenous PGCs present in embryonic day 12.5 (e12.5) mouse ovaries. Accordingly, Saitou and colleagues aggregated in vitro-derived PGCLCs specified 3, 4, and 6 days after initiation of ESC differentiation with dispersed e12.5 ovarian tissue and then transplanted the aggregates under the ovarian bursal sacs of adult female mice. The PGCLCs subsequently developed into germinal vesicle stage oocytes that, following harvesting and in vitro maturation, underwent in vitro fertilization. The resultant 2-cell stage embryos were transferred to pseudopregnant female mice, of which a low percentage survived gestation and were delivered as apparently healthy pups.2
Prior to the publication of this most recent report from Saitou and colleagues,2 many other scientists have been testing the concept that cells other than PGCs are capable of generating fertilization-competent eggs, 2 of which succeeded using female germ line or oogonial stem cells purified from adult mouse ovaries.3–6 This new study nonetheless represents a major milestone in both reproductive and stem cell biology for 2 principal reasons. The first, and most obvious, is the demonstration that mouse ESCs and iPSCs can actually be differentiated, albeit at low frequency, into oocytes capable of fertilization, embryogenesis, and development to live offspring. The second is that PGCLCs, which resemble endogenous PGCs in fetal gonads, require interaction with developmentally matched embryonic ovary somatic cells to realize their full potential in vivo. Indeed, prior attempts at generating competent oocytes from mouse ESC-derived germ cells transplanted into ovaries have failed, most likely because the PGCLCs were introduced into postnatal, rather than embryonic, mouse ovary tissue.7 In order to provide the microenvironmental cues necessary for oogenesis, folliculogenesis, and ultimately egg formation from PGCLCs, a source of developmentally matched (embryonic) ovarian somatic cells is required. Unfortunately, the majority of published articles describing the differentiation of ESCs into ovarian cell types have focused almost exclusively on specification of germ cells,8–10 with derivation of developmentally matched ovarian somatic cells comparatively understudied.11,12 As such, even with ancillary evidence that ESCs can differentiate in vitro into steroid hormone-producing cells that participate in the assembly of structures resembling follicles,1,8–10,12,13 there is a paucity of direct information regarding the derivation of granulosa cells from ESCs.
Follicle-like structures formed by mouse ESCs in vitro consist of a single oocyte-like cell that can grow as large as 70 µm in diameter surrounded by one or more layers of tightly adherent somatic cells.12 Analogous to what is observed during normal follicle formation within the ovary, somatic cells within ESC-derived follicle-like structures are connected via intercellular bridges with their enclosed germ cells, which may serve to facilitate cell-to-cell interaction required for normal follicle development.14 Additionally, increased expression of steroidogenic pathway genes, along with estrogen secretion into the culture medium, occurs concomitant with the formation of follicle-like structures from ESCs in vitro.1,13 While these data collectively support the notion that somatic cells of in-vitro-derived follicle-like structures have features ultrastructurally and functionally similar to endogenous granulosa cells, isolation and characterization of these cells from differentiating ESCs have been difficult.11 Herein we describe the use of mouse ESCs engineered to express a Discosoma sp red (DsRed) fluorescent reporter, under regulatory control of the ovarian granulosa cell–specific Forkhead box L2 (Foxl2) gene promoter,15 to track granulosa cell specification, function, and fate in vitro and in vivo.
Materials and Methods
Animals and Cell Lines
Wild-type C57BL/6 female mice (Charles River Laboratories, Wilmington, Massachusetts) were used for all in vivo experiments. A mouse XX ESC line with germ cell lineage expression of green fluorescent protein (GFP) was generated from TgOG2 (▵PE-Pou5f1-Gfp) transgenic female mice using standard methods essentially as reported,1 whereas wild type mouse XY ESCs (v6.5) were from Novus Biologicals (Littleton, Colorado). All experiments involving mice described herein were reviewed and approved by the institutional animal care and use committee of Massachusetts General Hospital.
ESC Culture, Differentiation, and Live Cell Imaging
Undifferentiated ESCs were maintained on a mitotically inactivated mouse embryonic fibroblast (MEF) feeder layer in DMEM supplemented with 15% heat-inactivated ESC-grade fetal bovine serum (FBS; Invitrogen, Carlsbad, California), 1% sodium pyruvate, 1% nonessential amino acids, 1% penicillin–streptomycin (Invitrogen), 103 U/mL leukemia inhibitory factor (LIF; Millipore, Billerica, Massachusetts), and 0.1 mmol/L β-mercaptoethanol (Sigma-Aldrich, St Louis, Missouri). For differentiation, ESCs were separated from MEFs by differential adhesion and cultured with 15% FBS in the absence of LIF on gelatin-coated plates in a monolayer, as described.1 Live cell imaging was performed using a Nikon Eclipse TE2000-S inverted fluorescent microscope and SPOT imaging software (Diagnostic Instruments, Sterling Heights, Michigan).
Plasmids, Vector Expression in ESCs, and Isolation of DsRed-Positive Cells
A 739-bp region of the Foxl2 gene promoter, conserved between mouse and human, was identified using Genome Vista,16,17 isolated from mouse genomic DNA (see Table 1 for primer sequence information), and cloned into the pDsRed2-1 vector (Clontech, Mountain View, California) or a pLenti6 lentiviral construct containing the complete open reading frame of DsRed (Gateway Lentiviral System; Invitrogen). Promoter activity and specificity were verified using mouse granulosa cells as a positive control and 293 cells (Invitrogen) as a negative control. For detection of Foxl2 gene promoter-driven DsRed expression, undifferentiated ESCs were stably transfected with the Foxl2-pDsRed2-1 construct via electroporation, followed by clonal selection and expansion. Alternatively, ESCs were virally transduced following initiation of differentiation using viral supernatant produced by 293 cells transfected with the Foxl2-DsRed lentiviral construct (pLenti6-Foxl2-DsRed). Cells were analyzed for expression of DsRed by fluorescence microscopy and isolated by fluorescence-activated cell sorting (FACS).
Table 1.
Sequence of Forward (F) and Reverse (R) Oligonucleotide Primers Used for PCR-Based Amplification of the Mouse Foxl2 Gene Promoter (Upstream Noncoding Region Derived From Ensembl Gene ID ENSMUSG00000050397) or Expression Analysis of the Indicated Genes.
| Gene | Accession No. | Primer Sequence (5’-3’) |
|---|---|---|
| Foxl2 gene promoter | F: CATGGATCCTTTCACCTGAAAGCTGCC R: GGCCATGGTGACAAAAGCCGG | |
| Foxl2 | BC137811 | F: AAGCCCCCGTACTCGTACGTGGCGCTCATC R: GTAGTTGCCCTTCTCGAACATGTC |
| Wnt4 | M89797 | F: AGAAGCTCAAAGGCCTGATCCAGA R: TCCACAAAGGACTGTGAGAAGGCT |
| Nr5a1 | AF511594 | F: GCCAGGAGTTCGTCTGTCTC R: ACCTCCACCAGGCACAATAG |
| Fst | X83377 | F: AGAGGAAATGTCTGCTTCCG R: CACCTCTCTTCAGTCTCCTG |
| Kitl | M64262 | F: CTATGTCGCCGGGATGGATG R: CCTTTGCGGCTTTCCTATTACT |
| Fshr | AF095642 | F: TGGATGTCATCACTGGCTGTGTCA R: ATTCTGGAAGGCCTCAGGGTTGAT |
| Amh | AY911505 | F: TTGCTGAAGTTCCAAGAGCCTCCA R: GAAACAGCGGGAATCAGAGCCAAA |
| Cyp19a1 | D00659 | F: TCATGGGCCTCCTTCTCCTGATTT R: AAGGGCGAATTGTTCTCCAAAGGC |
| StAR | L36062 | F: AAGCTGTGTGCTGGAAGCTCCTAT R: TTCCTTCTTCCAGCCTTCCTGGTT |
| Lhr | M81310 | F: CAATGCAGTGGCCTTTGTCGTCAT R: TGAAATGCCTTCGTGAACACTGCG |
| β-actin | X03672 | F: GATGACGATATCGCTGCGCTG R: GTACGACCAGAGGCATACAGG |
Abbreviations: Foxl2, Forkhead box L2; Fst, follistatin; Amh, anti-Müllerian hormone; Fshr, follicle-stimulating hormone receptor; Wnt4, Wingless-related MMTV integration site 4; Kitl, Kit ligand; Nr5a1, nuclear receptor subfamily 5 group A member 1; Lhr, luteinizing hormone receptor; Cyp19a1, cytochrome P450 family 19 subfamily a polypeptide 1; StAR, steroidogenic acute regulatory protein.
For FACS, differentiating ESCs were removed from the plate by either 0.25% trypsin-EDTA (prior to day 10 of differentiation) or manual scraping, and then incubated with 800 U/mL of type IV collagenase (Worthington, Lakewood, New Jersey) with gentle dispersion for 15 minutes followed by incubation with 0.25% trypsin-EDTA for 10 minutes to obtain single-cell suspensions (after day 10 of differentiation). Cells were prepared for FACS by resuspension in 1× concentrated phosphate-buffered saline (PBS) containing 0.1% FBS and filtration (35-µm pore size). The cells were analyzed and sorted using a FACSAria flow cytometer (BD Biosciences, San Jose, California) at the Harvard Stem Cell Institute Flow Cytometry Core Facility (Boston, Massachusetts). Collected cells were used for analysis of gene expression, replated for steroid hormone assays after short-term culture, or used for intraovarian transplantation experiments.
Reverse Transcriptase–Polymerase Chain Reaction Analysis of Gene Expression
Total RNA was isolated from 200 FACS-purified DsRed-expressing cells at each time point postdifferentiation using the RNeasy Micro kit (Qiagen, Valencia, California) and was reverse transcribed using the Reverse Transcription System (Promega, Madison, Wisconsin). Samples were then analyzed by conventional polymerase chain reaction (PCR) to determine whether and when each indicated messenger RNA (mRNA) transcript first became detectable at different points after the induction of ESC differentiation. The genes selected represent a spectrum of accepted markers associated with the early specification of granulosa cells and their subsequent differentiation. Amplification conditions were specific for each primer pair (Table 1) and included an initial denaturation step for 3 minutes at 94°C followed by 40 cycles of denaturation at 94°C (30 seconds), annealing at 51°C–60°C (30 seconds), and extension at 68°C (60 seconds) using Platinum Taq DNA polymerase (Invitrogen). All products were sequenced to confirm identity.
Hormone Assays
Estradiol and progesterone concentrations were measured in culture medium from FACS-purified Foxl2-DsRed-positive cells that had been replated and cultured for 24, 48, or 72 hours in the presence of PBS (vehicle), 100 ng/mL follicle-stimulating hormone (FSH; NIDDK, NIH, Bethesda, Maryland) or 1 mmol/L 8-bromoadenosine-3',5'-cyclic monophosphate (8-br-cAMP; Sigma-Aldrich). Androgen substrate necessary for aromatization to estrogen was provided by the presence of heat-inactivated 15% FBS in all cultures, which contained 0.92 pg/mL androgen (mean of 56 lots of FBS tested). The estradiol enzyme-linked immunosorbent assay (ELISA) was from Alpco (Salem, New Hampshire), and the progesterone ELISA was from DRG International (Mountainside, New Jersey). All assays were performed according to the manufacturer’s guidelines.
Ovarian Injections and Kidney Capsule Transplants
Following the differentiation of Foxl2-DsRed-expressing ESCs for 12 days, FACS was used to isolate DsRed-positive cells. For each experiment, 200 to 500 DsRed-positive cells were microinjected into a single neonatal (day 2-4 postpartum) wild-type mouse ovary using a Pneumatic PicoPump (World Precision Instruments, Sarasota, Florida). Injected ovaries were then transplanted under kidney capsules of ovariectomized wild-type female mice at 6 weeks of age. At 8 days and 2 weeks posttransplantation, the grafted ovaries were removed and fixed in 4% paraformaldehyde (PFA) for analysis.
Immunofluorescence Imaging
Fixed ovaries were embedded in paraffin, serially sectioned, mounted on slides and de-waxed in xylenes, followed by hydration in a graded ethanol series. Antigen retrieval was performed by boiling the slides for 5 minutes in sodium citrate (pH 6.0), followed by blocking in TNK buffer (0.1 mol/L Tris, 0.55 mol/L NaCl, 0.1 mmol/L KCl, 1% goat serum, 0.5% bovine serum albumin, and 0.1% Triton-X in PBS), incubated with the desired primary antibody (1:100 dilution) overnight at 4°C, and fluor-conjugated secondary antibody (1:250 dilution, Alexa Fluor-488 or -568; Invitrogen) at 20°C for 1 hour. Primary antibodies used were mouse anti-Dazl antibody from Serotec (MCA2336; Raleigh, North Carolina) and rabbit anti-RFP antibody for detection of DsRed from Abcam (ab62341; Cambridge, Massachusetts). Fluorescence image analysis was performed using a Nikon Eclipse TE2000-S inverted fluorescent microscope and SPOT imaging software (Diagnostic Instruments).
Data Analysis
All experiments were independently replicated at least 3 times using different cells or mice. Quantitative data (mean ± standard error of the mean [SEM] of combined replicates) were analyzed by 1-way analysis of variance followed by Tukey's post hoc test using PRISM software.
Results
Follicle-Like Structure Formation in Differentiating ESC Cultures
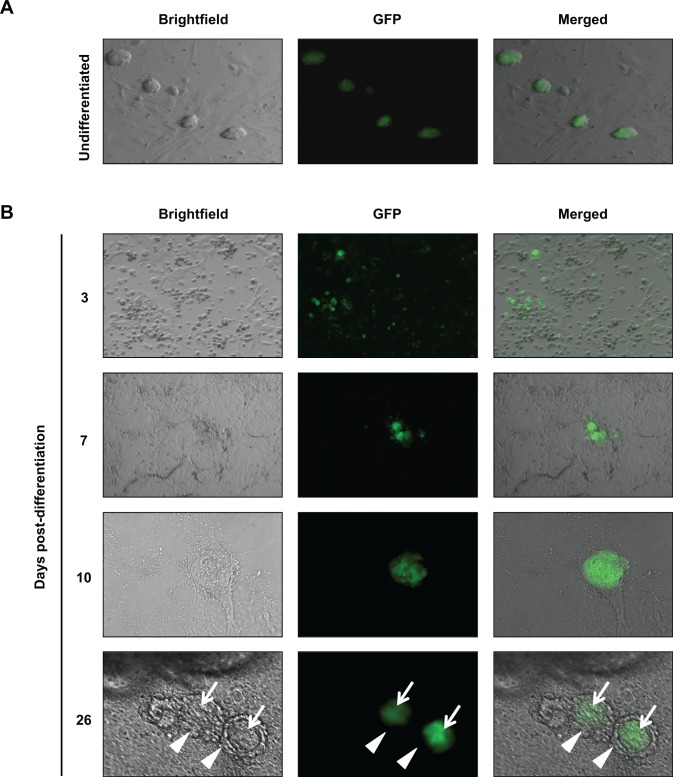
Expression of GFP was detected in undifferentiated TgOG2 ESC colonies (Figure 1B), whereas the initiation of differentiation by removal of MEFs and LIF resulted in a progressive loss of widespread GFP expression over the next 3 to 7 days (Figure 1B). This was followed by the formation of distinct colonies comprising GFP-positive cells, between days 7 and 26 after the initiation of differentiation (Figure 1B). Follicle-like structures consisting of a single large GFP-positive oocyte-like cell surrounded by somatic cells were detected between days 16 and 40 of differentiation (Figure 1B), similar to that reported by others.1,7
Figure 1.
Differentiation of TgOG2 ESCs in vitro. A and B, Live cell imaging of GFP expression in TgOG2 ESC cultures during in vitro differentiation. Note examples of ESC-derived follicle-like structures at day 26, each consisting of a large GFP-positive germ cell (white arrows) surrounded by one or more concentric layers of tightly adherent non-GFP-expressing somatic cells (white arrowheads). ESCs indicates embryonic stem cells; GFP, green fluorescent protein. Please see online version for color reference.
Granulosa Cell Specification and Isolation
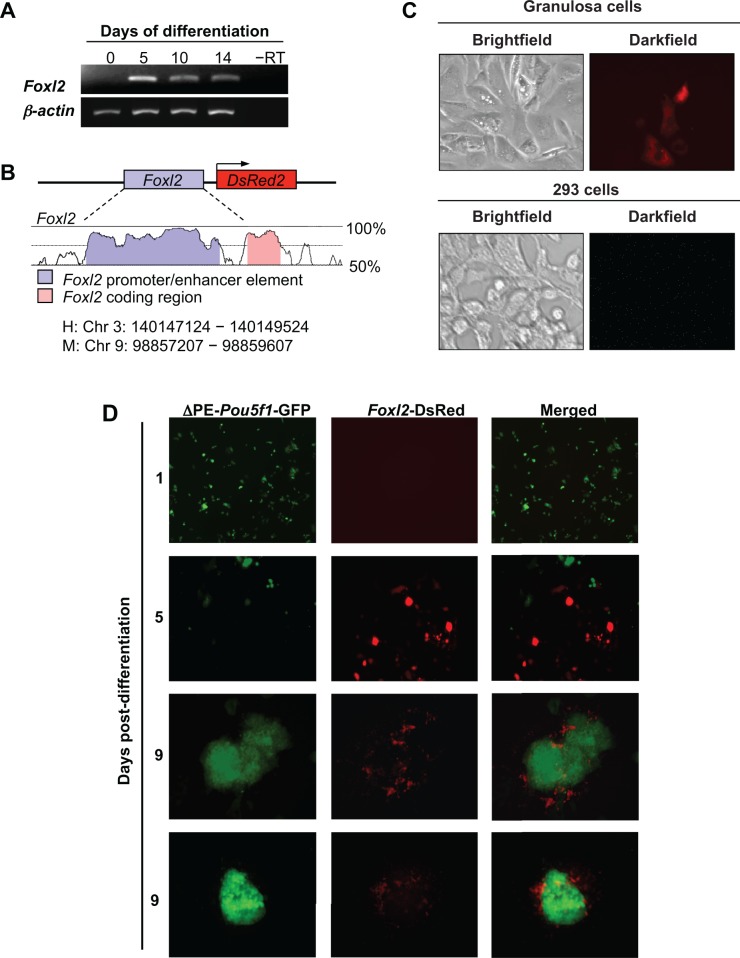
To identify and track ovarian somatic cells in differentiating ESC cultures, we first mapped the expression of the early granulosa cell marker, Foxl2, which revealed activation of the gene by day 5 (Figure 2A) but not at earlier time points (data not shown). We then designed a DsRed expression vector under control of the Foxl2 gene promoter (Figure 2B) and confirmed the lineage specificity of its activation through analysis of granulosa cells (positive control) and 293 cells (negative control) engineered to express the reporter (Figure 2C). We next stably introduced the Foxl2-DsRed vector into TgOG2 ESCs and then removed MEFs and LIF to induce differentiation. Cells positive for DsRed expression were absent on day 1, but were observed as early as day 5, of differentiation. By day 9, DsRed-expressing cells were found in close association with GFP-expressing colonies (Figure 2D). Notably, DsRed-expressing cells were nearly exclusively colocalized with GFP-expressing cell clusters at this time (Figure 2D).
Figure 2.
Differentiation of TgOG2 ESCs co-expressing Foxl2-DsRed. A, Foxl2 expression is observed in differentiating ESCs but not in undifferentiated cells (day 0). β-actin, positive PCR control gene for sample loading; –RT, RNA sample lacking reverse transcriptase. B, Conserved elements of the human (H) FOXL2 and mouse (M) Foxl2 gene promoters. C, DsRed is expressed in mouse granulosa cells but not in 293 cells, following transfection with the Foxl2-DsRed construct. D, Initiation of differentiation of TgOG2 ESCs co-expressing Foxl2-DsRed results in the gradual loss of GFP expression (days 1 to 5), with minimal GFP detected 5 days after initiation of differentiation; the GFP signal returns in a subset of cells by day 9. In parallel, DsRed-positive cells are absent on day 1 but are observed by 5 days following differentiation. By day 9, Foxl2-DsRed-expressing cells are nearly exclusively colocalized with GFP-expressing cell clusters. ESCs indicates embryonic stem cells; GFP, green fluorescent protein; Foxl2, Forkhead box L2; PCR, polymerase chain reaction; DsRed, Discosoma sp red. Please see online version for color reference.
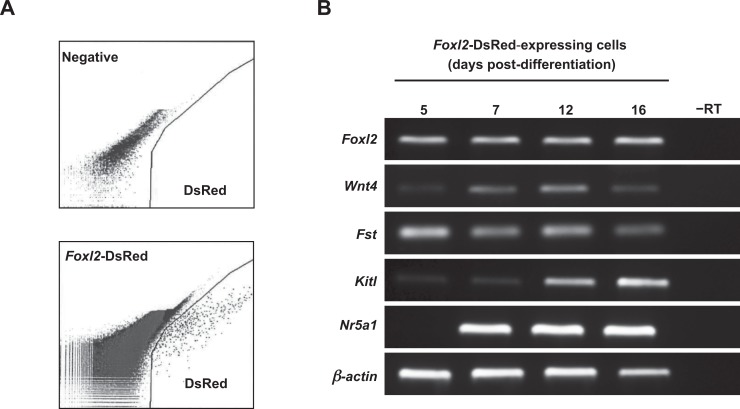
Gene expression analysis of DsRed-positive cells isolated by FACS at day 5 of differentiation (Figure 3A) revealed a distinct somatic cell gene expression profile consistent with the presence of early stage ovarian granulosa cells (Figure 3B). The mRNAs detected were Foxl2, Wingless-related MMTV integration site 4 (Wnt4), follistatin (Fst), and Kit ligand (Kitl; also referred to as stem cell factor or Scf, or Steel factor). Expression of nuclear receptor subfamily 5 group A member 1 (Nr5a1; also referred to as steroidogenic factor 1 or Sf-1) became detectable by day 7 (Figure 3B).
Figure 3.
Primitive granulosa cell markers are expressed during specification of Foxl2-DsRed expressing cells in differentiating ESC cultures. A, DsRed-expressing cells are detectable by day 5 of differentiation and can be isolated by FACS (negative, parallel sorts of TgOG2 ECSs lacking Foxl2-DsRed). B, Expression of early granulosa cell markers in Foxl2-DsRed-expressing cells isolated by FACS at the indicated days of differentiation (–RT, RNA sample lacking reverse transcriptase; β-actin, positive PCR control gene for sample loading). ESCs indicates embryonic stem cells; FACS, fluorescence-activated cell sorting; Foxl2, Forkhead box L2; DsRed, Discosoma sp red.
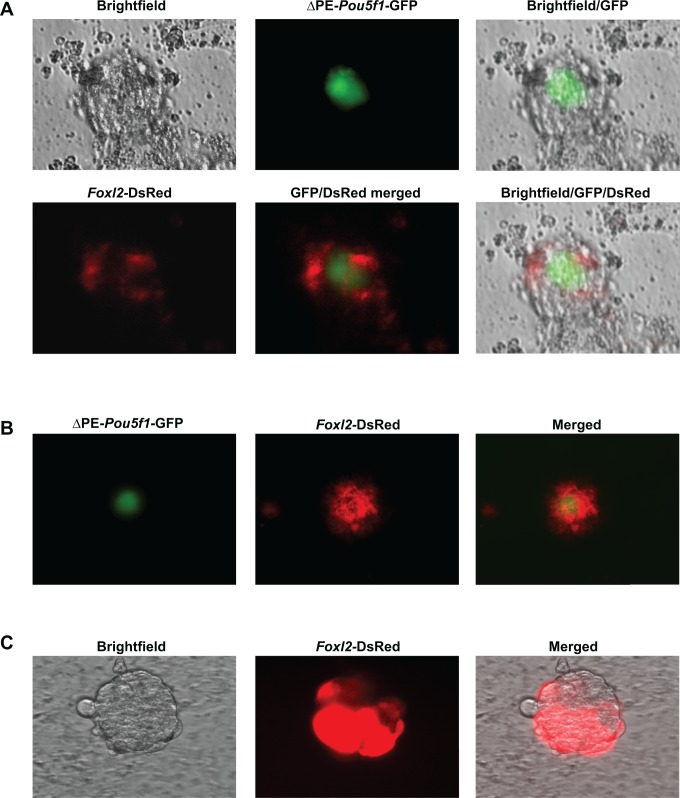
Beginning on day 16 and continuing through day 40 of differentiation, multidimensional follicle-like structures, each one consisting of a single large (25-50 µm) GFP-positive cell surrounded by DsRed-expressing cells, were observed by direct fluorescence (Figure 4A, B). To ensure that these observations were not specific to the TgOG2 ESC line, we confirmed that v6.5 ESCs transduced with Foxl2-DsRed formed the same follicle-like structures comprising a centrally located oocyte-like cell surrounded by DsRed-expressing somatic cells following the induction of differentiation for at least 16 days (Figure 4C).
Figure 4.
Live cell imaging of representative follicle-like structures derived from differentiating ESCs in vitro. A and B, Follicle-like structures formed in cultures of TgOG2 ESCs coexpressing Foxl2-DsRed at 16 days (A) and 40 days (B) following the initiation of differentiation. Note that in each case, ▵PE-Pou5f1-driven GFP expression is detectable in a single large cell surrounded by multiple Foxl2-DsRed-expressing cells. C, DsRed-expressing cells are detectable in follicle-like structures formed by v6.5 ESCs, transduced with Foxl2-DsRed, following 16 days of differentiation. ESCs indicates embryonic stem cells; GFP, green fluorescent protein;; Foxl2, Forkhead box L2; DsRed, Discosoma sp red. Please see online version for color reference.
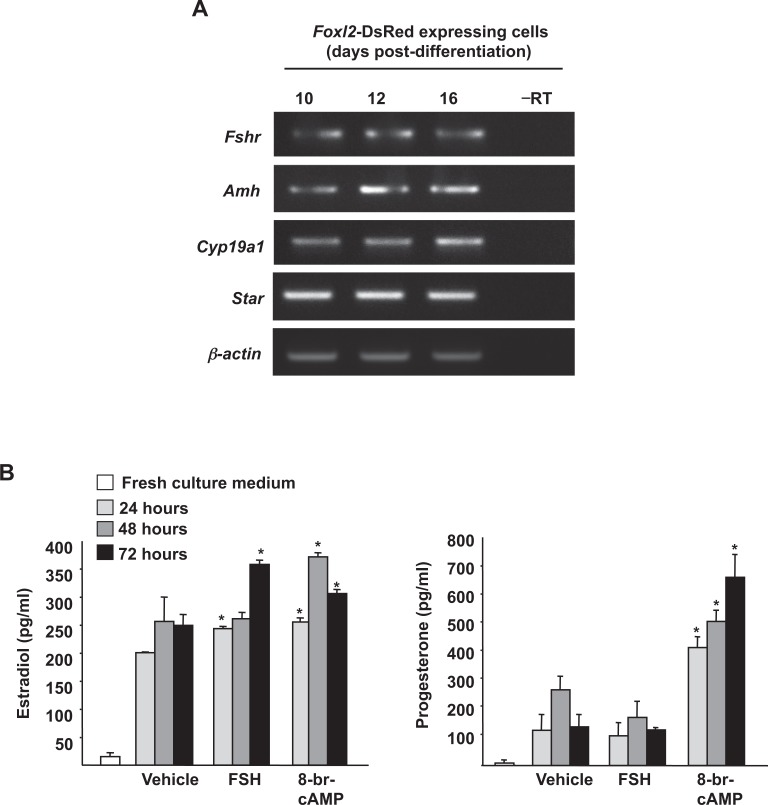
Gene expression analysis of DsRed-expressing cells isolated by FACS on day 10 of differentiation revealed the presence of multiple markers classically associated with differentiating granulosa cells, including follicle-stimulating hormone receptor (Fshr), anti-Müllerian hormone (Amh; also referred to as Müllerian-inhibiting substance or Mis), cytochrome P450 family 19 subfamily a polypeptide 1 (Cyp19a1; also referred to as aromatase), and steroidogenic acute regulatory protein (StAR; Figure 5A). Evaluation of steroidogenesis following subculture of DsRed-positive cells isolated on day 12 of differentiation revealed the presence of both estradiol and progesterone in the culture medium (Figure 5B). Moreover, treatment with either FSH or 8-br-cAMP led to a significant increase in estradiol production, confirming the presence of functional FSH receptors and cAMP-mediated signaling coupled with steroidogenesis in these cells; however, only 8-br-cAMP was able to significantly enhance progesterone production (Figure 5B).
Figure 5.
Foxl2-DsRed-expressing cells synthesize ovarian steroid hormones following isolation and culture. A, Foxl2-DsRed positive cells isolated from ESC cultures between days 10 and 16 after initiation of differentiation express markers of differentiating granulosa cells (–RT, RNA sample lacking reverse transcriptase; β-actin, positive PCR control gene for sample loading). B, Estradiol and progesterone production by FACS-purified Foxl2-DsRed positive cells (2 × 103 cells per well) maintained in culture for up to 3 days (FSH, 100 ng/mL; 8-br-cAMP, 1 mmol/L). Data are the mean ± SEM of 3 independent cultures (*P < .05 vs vehicle control). ESCs indicates embryonic stem cells; FACS, fluorescence-activated cell sorting; FSH, follicle-stimulating hormone; PCR, polymerase chain reaction; Foxl2, Forkhead box L2; DsRed, Discosoma sp red; RT, reverse transcriptase.
Intraovarian Transplantation of Presumptive Granulosa Cells
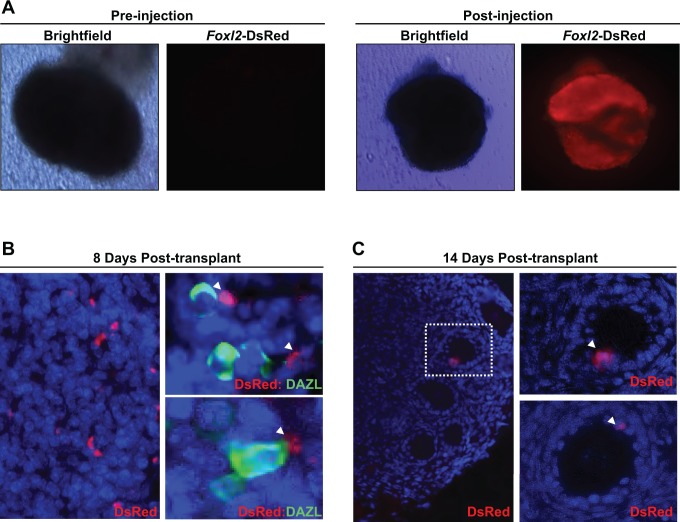
In a final set of experiments, Foxl2-DsRed-expressing ESCs were differentiated for 12 days. Cells positive for DsRed expression were then isolated by FACS and directly injected into ovaries removed from wild-type neonatal mice (Figure 6A). Injected ovaries were subsequently transplanted under the kidney capsules of ovariectomized wild-type female hosts. At 8 days posttransplantation, DsRed-expressing cells were found distributed throughout the stroma of the injected ovaries. Many of these cells were observed in close proximity to immature oocytes, as indicated by dual-immunofluorescence staining for DsRed and the oocyte marker Dazl (Deleted in azoospermia like; Figure 6B). At 14 days posttransplantation, DsRed-expressing cells were no longer observed in the stroma but were detected exclusively within the granulosa layer of growing follicles (Figure 6C).
Figure 6.
Immunofluorescent imaging of DsRed-expressing cells within ovarian tissue following engraftment under the kidney capsule. A, Wild-type neonatal ovary before and after injection of Foxl2-DsRed-expressing cells isolated from ESC cultures 12 days postdifferentiation. B, DsRed-expressing cells are present within the ovarian stroma at 8 days posttransplant (left); by dual immunofluorescence, these cells frequently associate with immature oocytes identified by expression of the oocyte marker Dazl (green; right panels). C, DsRed-expressing cells are found only in the granulosa cell layer of growing follicles at 14 days posttransplant. ESCs indicates embryonic stem cells; Foxl2, Forkhead box L2; DsRed, Discosoma sp red. Please see online version for color reference.
Discussion
Past reports indicate that somatic cells contained in follicle-like structures formed in cultures of differentiating ESCs have some characteristic features of endogenous ovarian-derived granulosa cells.1,12,13 To our knowledge, however, these cells have not been previously isolated. Using a Foxl2 promoter-driven reporter system, we have succeeded in purifying what appear, by lineage-specific gene expression profiling and functional testing (FSH responsiveness in vitro, incorporation into the granulosa cell layer of follicles in vivo), to be granulosa cells from ESC cultures during the earliest stages of specification. However, it bears mention that the approach employed can be improved on since early granulosa cell markers could sometimes be detected in the negative (non-DsRed expressing) cell population. This may reflect our FACS-based exclusion of a fraction of ESC-specified granulosa cells with a level of Foxl2-driven reporter expression that was too low to accurately identify and sort as positive cells.
The main problems faced in the purification and study of ESC-derived ovarian somatic cells are the vast heterogeneity of somatic cell types present in differentiating ESC cultures along with the reported low frequency of ovarian follicle-like structure formation.1 It is perhaps not surprising that these structures are rare, as ovarian follicles are comprised of cell types derived from distinct embryonic lineages. During mouse embryogenesis, PGC specification from pluripotent epiblast cells occurs around e7. Although PGCs are motile at this stage, they are retained in the hindgut until e9.5, at which time the cells initiate an active process of migration to the genital ridges over the next 2 days.18 Ovarian follicular somatic cells, on the other hand, are thought to be derived from the mesenchyme of the genital ridges and begin to proliferate only after PGCs migrate to and colonize the embryonic gonads.
While this developmental heterogeneity likely contributes to the rarity of follicle-like structure formation in ESC cultures, use of germ cell–selective fluorescent reporters has facilitated progress in this field. Among the most widely studied is a germ cell lineage reporter with GFP expression driven by a fragment of the mouse POU domain class 5 transcription factor 1 (Pou5f1; also known as octamer-binding transcription factor 4 or Oct4) gene promoter in which the epiblast-specific proximal enhancer region has been inactivated.1,19 The ESCs generated from transgenic TgOG2 mice expressing this reporter can be reliably differentiated into GFP-positive oocyte-like cells in vitro.1,7 Moreover, when GFP-positive cells are isolated from differentiating TgOG2 ESC populations and transplanted into wild-type mouse ovaries, immature follicles containing GFP-positive oocytes can be detected.7
In agreement with the results of others,1,7,12,13 our findings confirm that follicle-like structures spontaneously form in monolayer cultures of mouse ESCs by day 16 of differentiation and continue to form through day 40. While the incidence of follicle-like structure formation was variable across the replicate cultures analyzed, GFP-expressing germ cells were consistently detected in all cultures. However, in ESCs expressing the Foxl2-DsRed reporter, we observed that approximately 20% of the more than 200 differentiating ESC cultures tested in this study failed to show the presence of any DsRed-positive cells. In addition, only those cultures containing cells expressing DsRed by day 5 of differentiation eventually generated follicle-like structures (data not shown). This indicates that not only do both cell types (germ cells and granulosa cells) need to be specified and present in these cultures for follicle-like structures to form but also the kinetics of granulosa cell and germ cell specification are critical. This conclusion is supported by in vivo observations with mice, showing that germ cell and granulosa precursor cell synchronization during fetal life is required for normal folliculogenesis.20–22
Our ability to isolate Foxl2-DsRed-expressing cells from all other cell types that arise in differentiating ESC cultures allowed us to perform a detailed assessment of changes in expression of known granulosa cell markers with the in-vitro progression of differentiation. Consistent with an early-stage or pregranulosa cell phenotype, DsRed-expressing cells isolated on day 5 of ESC differentiation were positive for Foxl2, Wnt4, Fst, and Kitl. While detectable expression of Nr5a1 was comparatively delayed until day 7 of differentiation, this agrees with results from others using differentiating mouse ESCs11 as well as with expression patterns of these genes observed in vivo.23,24 After 10 days of differentiation, the gene expression pattern in isolated DsRed-expressing cells began a transition to one associated with slightly more differentiated granulosa cells, as revealed by the activation of Fshr and Amh gene expression. Notably, expression of luteinizing hormone receptor (Lhr) was never detected in our ESC cultures at any point postdifferentiation, and isolated Foxl2-DsRed-positive cells were not responsive to treatment with LH as measured by either estradiol or progesterone output (data not shown). This observation, combined with our findings that FSH significantly increases estrogen production by these cells, collectively indicate that the granulosa cells obtained from differentiating ESCs after day 10 resemble granulosa cells present in early secondary stage follicles.
To map the in vivo fate of these cells in a more natural setting by intraovarian transplantation, we used cultures differentiated for 12 days before isolation of Foxl2-DsRed-expressing cells to maximize the number of cells obtained from each culture. This time point is several days prior to the onset of follicle-like structure formation, and thus the DsRed-positive cells obtained would still resemble early stage pregranulosa cells. In addition, an FSH-responsive steroidogenic pathway can be activated in Foxl2-expressing cells at this stage of collection, solidifying their identity as granulosa cells. Even after waiting until day 12 postdifferentiation, a current limitation of this system for in vivo studies remains the very low frequency at which granulosa cells are specified from cultures of differentiating ESCs. In fact, 10 or more cultures of differentiating ESCs in 100 mm plates had to be pooled just to obtain the 200 to 500 DsRed-positive cells by FACs that were used for each intraovarian injection.
Despite the relatively low number of cells available for use, the injected DsRed-positive cells were clearly observed to associate with oocytes within a week after injection and then become incorporated, and exclusive localized, into the granulosa cell layer of immature growing follicles 2 weeks after transplantation These in vivo outcomes align well with our in vitro findings that Foxl2-DsRed-expressing cells, once specified, follow a progressive pattern of gene expression consistent with granulosa cell differentiation, not unlike what is observed in the developing gonad. Furthermore, these cells actively synthesize steroid hormones under basal culture conditions and elevate estradiol biosynthesis in response to FSH stimulation. Taken together, these data indicate that use of a Foxl2 gene promoter-driven fluorescent reporter system facilitates purification and study of granulosa cells at progressive stages of differentiation from ESC cultures. The ability to now purify this important ovarian somatic cell lineage at the earliest stages of specification from differentiating ESCs may provide a means to more rapidly identify the factors and cell-to-cell interactions provided specifically by the embryonic ovarian somatic environment that instruct PGCLCs to differentiate into oocytes capable of maturation into fertilization-competent eggs.2
Acknowledgments
We thank Laura Prickett-Rice and Kat Folz-Donahue of the Harvard Stem Cell Institute Flow Cytometry Core Facility for excellent technical assistance, and J. R. Mann and K. J. MacLaughlin for providing TgOG2 transgenic mice.
Footnotes
Authors' Note: The authors of this article performed all of the work while appointed at Massachusetts General Hospital and Harvard Medical School. Yvonne A. R. White is currently at Leica Biosystems, Danvers, MA, USA; Yuichi Niikura is currently at the Department of Pharmacotherapy, Research Institute of Pharmaceutical Sciences, Musashino University, Tokyo, Japan; Sorapop Kiatpongsan is currently at the Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; and Ho-Joon Lee is currently at the Center for Human Reproduction, New York, NY, USA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.C.W., Y.A.R.W., Y.N., S.K. and H.J.L. declare no competing interests; J.L.T. declares interest in intellectual property described in U.S. Patent 7,955,846 and is a co-founder of OvaScience, Inc. (Cambridge, MA).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a United States Department of Agriculture National Research Initiative Competitive Grant (2007-35203-18085 to D.C.W.), a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809 to D.C.W.), a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279 to J.L.T.), and a grant from the Glenn Foundation for Research in the Biological Mechanisms of Aging (to J.L.T.).
References
- 1.Hübner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–1256. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975. [DOI] [PubMed] [Google Scholar]
- 3.Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Yang Z, Yang Y, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141. [DOI] [PubMed] [Google Scholar]
- 5.White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods DC, White YAR, Tilly JL. Purification of oogonial stem0 cells from adult mouse and human ovaries: an assessment of the literature and a view towards the future. Reprod Sci. 2013;20(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet. 2009;18(22):4376–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholas CR, Chavez SL, Baker VL, Reijo Pera RA. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr Rev. 2009;30(3):264–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko K, Schöler HR. Embryonic stem cells as a potential source of gametes. Semin Reprod Med. 2006;24(5):322–329. [DOI] [PubMed] [Google Scholar]
- 10.Nagano MC. In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod. 2007;76(4):546–551. [DOI] [PubMed] [Google Scholar]
- 11.Salvador LM, Silva CP, Kostetskii I, Radice GL, Strauss JF III. The promoter of the oocyte-specific gene, Gdf9, is active in population of cultured mouse embryonic stem cells with an oocyte-like phenotype. Methods. 2008;45(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Psathaki OE, Hübner K, Sabour D, et al. Ultrastructural characterization of mouse embryonic stem cell-derived oocytes and granulosa cells. Stem Cells Dev. 2011;20(12):2205–2215. [DOI] [PubMed] [Google Scholar]
- 13.Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24(8):1931–1936. [DOI] [PubMed] [Google Scholar]
- 14.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt D, Ovitt CE, Anlag K, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131(4):933–942. [DOI] [PubMed] [Google Scholar]
- 16.Bray N, Dubchak I, Pachter L. AVID: A global alignment program. Genome Res. 2003;13(1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couronne O, Poliakov A, Bray N, et al. Strategies and tools for whole-genome alignments. Genome Res. 2003;13(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molyneaux KA, Stallock J, Schaible K, Wyllie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498. [DOI] [PubMed] [Google Scholar]
- 19.Yeom YI, Fuhrmann G, Ovitt CE, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122(3):881–894. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas CR, Haston KM, Reijo Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev Biol. 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei L, Zhang H, Jin S, et al. Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208(3):640–647. [DOI] [PubMed] [Google Scholar]
- 22.Qing T, Liu H, Wei W, et al. Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod. 2008;23(1):54–61. [DOI] [PubMed] [Google Scholar]
- 23.Hatano O, Takayama K, Imai T, et al. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120(10):2787–2797. [DOI] [PubMed] [Google Scholar]
- 24.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1996;1(7):663–671. [DOI] [PubMed] [Google Scholar]