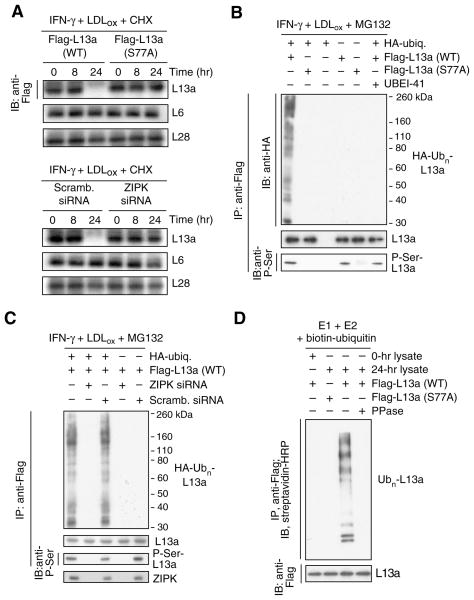
Figure 2. Phosphorylation-dependence of L13a ubiquitinylation and degradation.
(A) L13a degradation is phosphorylation-dependent. U937 cells were transfected with plasmids encoding Flag-tagged wild-type (WT) or phospho-null (S77A) L13a. After recovery, cells were treated with IFN-γ plus LDLox in the presence of cycloheximide (CHX) to inhibit de novo synthesis. Lysates were immunoblotted with anti-Flag, -L6, and -L28 antibodies (top 3 panels). Cells were transfected with ZIP kinase (or scrambled) siRNA and then incubated and immunoblotted as above (bottom 3 panels).
(B) L13a poly-ubiquitinylation is phosphorylation-dependent. U937 cells were co-transfected with plasmids encoding HA-ubiquitin and Flag-tagged wild-type or S77A mutant L13a. After recovery, cells were treated with IFN-γ plus LDLox and with MG132 and UBEI-41 as in Figure 1H. L13a in cell lysates was immunoprecipitated with anti-Flag antibody, and immunoblotted with anti-HA, -L13a, and -phospho-Ser antibodies.
(C) Poly-ubiquitinylation requires ZIP kinase (ZIPK). U937 cells were co-transfected with plasmids encoding HA-ubiquitin and Flag-L13a, and with ZIPK (or scrambled) siRNA. Cells were treated as above, immunoprecipitated with anti-Flag antibody, and immunoblotted with anti-HA (top), anti-L13a (second panel), or anti-phospho-Ser (third panel) antibodies. ZIPK was detected in 16 hr lysates by immunoblot with anti-ZIPK antibody (bottom).
(D) L13a phosphorylation is required for E3 ligase recognition. Flag-tagged L13a was incubated with biotin-ubiquitin and a reconstituted ubiquitinylation system containing recombinant E1 and E2 and lysate from cells treated with IFN-γ for 24 hr (or 0 hr as negative control) to provide E3 ligase and L13a kinase activity.