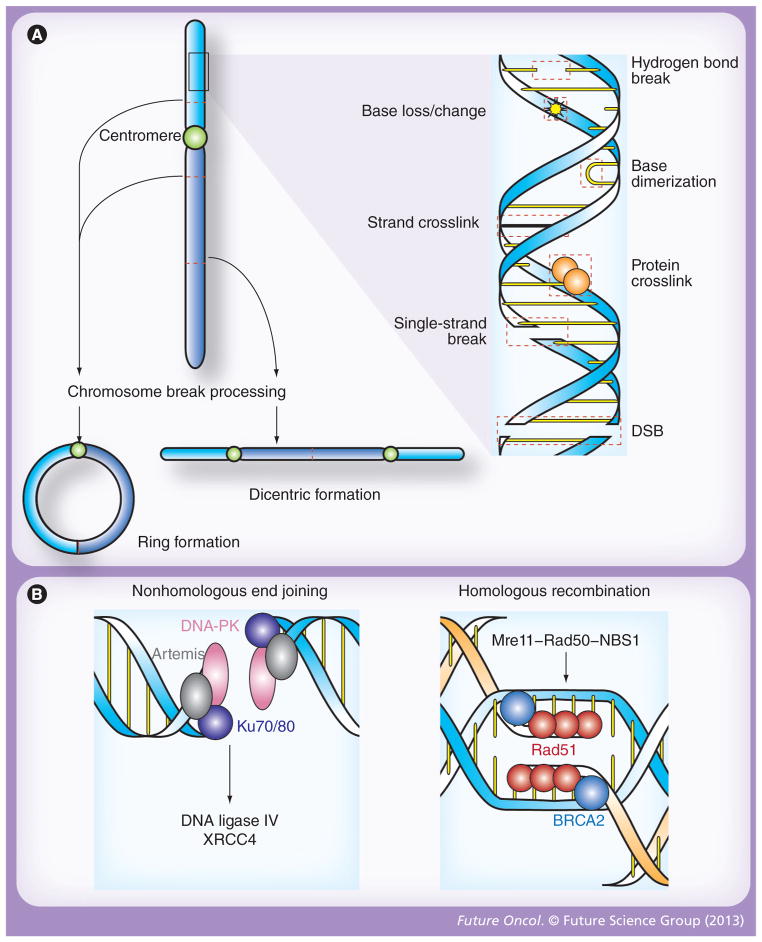
Figure 1. Patterns of DNA damage and double-stranded break repair following ionizing radiation.
(A) Radiation induces molecular damage, both directly and through the formation of radical oxygen species. At the level of the double helix, these changes including loss, alteration or dimerization of nitrogenous bases; crosslinkage of associated proteins or opposing DNA strands; and breakage of hydrogen bonds as well as one or both strands of DNA (right). While DSBs are the primary cytotoxic lesion associated with radiation, each of these changes activates distinct repair mechanisms, and, if significant, may result in chromosomal aberrations that are also processed by unique molecular repair machinery (left). In this regard, two break points within a single chromatid may fuse to form a ring structure (bottom left), while the union of opposing broken chromatids may result in dicentric formations (bottom right). Stable translocations, deletions and other nonlethal chromosomal aberrations may also occur after exposure to ionizing radiation, but similar to DSBs, ring and dicentric chromosomes lead to cell death via mitotic catastrophe. (B) DSBs are the principle cytotoxic lesion induced by ionized radiation, and their repair is primarily governed by nonhomologous end joining and homologous recombination [9]. In nonhomologous end joining (left), broken ends are recognized by the Ku70/80 heterodimer, which then recruits DNA-PK and artemis. This protein complex serves as a docking site for DNA ligase IV, and its cofactor XRCC4, which catalyze end joining. Homologous recombination (right) is initiated when the Mre11–Rad50–NBS1 complex recognizes the ends of a DSB. BRCA2 then loads Rad51 onto the processed ends, which triggers homologous strand invasion and subsequent ligation of the broken ends. DSB: Double-strand break.