Abstract
Stress hormones released by an experience can modulate memory strength via the basolateral amygdala, which in turn acts on sites of memory storage such as the cerebral cortex [McGaugh, J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28]. Stimuli that acquire behavioral importance gain increased representation in the cortex. For example, learning shifts the tuning of neurons in the primary auditory cortex (A1) to the frequency of a conditioned stimulus (CS), and the greater the level of CS importance, the larger the area of representational gain [Weinberger, N. M. (2007). Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory, 14(1–2), 1–16]. The two lines of research suggest that BLA strengthening of memory might be accomplished in part by increasing the representation of an environmental stimulus. The present study investigated whether stimulation of the BLA can affect cortical memory representations. In male Sprague–Dawley rats studied under urethane general anesthesia, frequency receptive fields were obtained from A1 before and up to 75 min after the pairing of a tone with BLA stimulation (BLAstm: 100 trials, 400 ms, 100 Hz, 400 μA [±16.54]). Tone started before and continued after BLAstm. Group BLA/1.0 (n = 16) had a 1 s CS–BLAstm interval while Group BLA/1.6 (n = 5) has a 1.6 s interval. The BLA/1.0 group did develop specific tuning shifts toward and to the CS, which could change frequency tuning by as much as two octaves. Moreover, its shifts increased over time and were enduring, lasting 75 min. However, group BLA/1.6 did not develop tuning shifts, indicating that precise CS–BLAstm timing is important in the anesthetized animal. Further, training in the BLA/1.0 paradigm but stimulating outside of the BLA did not produce tuning shifts. These findings demonstrate that the BLA is capable of exerting highly specific, enduring, learning-related modifications of stimulus representation in the cerebral cortex. These findings suggest that the ability of the BLA to alter specific cortical representations may underlie, at least in part, the modulatory influence of BLA activity on strengthening long-term memory.
Keywords: Auditory cortex, Frequency receptive field, Memory, Tuning shift
1. Introduction
It is well known that memories for emotional events are more robust than memories for neutral events (McGaugh, 2004). For example, stimuli that are rated as emotionally salient are better remembered than stimuli that are rated as neutral (Bradley, Greenwald, Petry, & Lang, 1992; Cahill, Prins, Weber, & McGaugh, 1994; Kleinsmith, Kaplan, & Tarte, 1963; Sharot & Yonelinas, 2008). Many studies in both the human and animal literatures have established that post-training increases of stress can also enhance memory consolidation (Cahill & Alkire, 2003; Cahill, Gorski, & Le, 2003; Hui, Hui, Roozendaal, McGaugh, & Weinberger, 2006; Kim, Lee, Han, & Packard, 2001; McCarty & Gold, 1981; Shors, 2001). Also, post-training systemic administration of stress hormones, such as corticosterone and epinephrine, is sufficient to enhance memory consolidation (Gold & van Buskirk, 1975; Gold, van Buskirk, & McGaugh, 1975; Hui et al., 2004; Kety, 1972; McGaugh & Roozendaal, 2002; Ogren, Archer, & Ross, 1980; Ridley, Haystead, Baker, & Crow, 1981; Roozendaal, Portillo-Marquez, & McGaugh, 1996).
Many lines of evidence have implicated the basolateral amygdala (BLA) as a substrate for stress-related modulation of memory. For example, post-training stimulation of the BLA is sufficient to induce enhanced memory consolidation for the training experience (Bergado, Rojas, Capdevila, González, & Almaguer-Melian, 2006; Gold, Hankins, Edwards, Chester, & McGaugh, 1975). Further, post-training administration of stress hormones into the BLA enhances memory consolidation in a variety of tasks (Fekete, Balázs, Telegdy, & Schally, 1985; Gallagher & Kapp, 1981; Gallagher, Kapp, Musty, & Driscoll, 1977; Hui et al., 2004; Hui et al., 2006; Introini-Collison, Nagahara, & McGaugh, 1986; Liang, Juler, & McGaugh, 1986; Roozendaal, Castello, Vedana, Barsegyan, & McGaugh, 2008; Yang, Chao, & Lu, 2006). Third, human imaging studies have also linked enhanced memory consolidation to amygdala activation. For example, PET and fMRI studies have shown that the level of activation in the BLA at the time of experiencing either negative or positive emotional experiences predicts the strength of memory at the time of recall (Cahill et al., 1996; Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Hamann, Ely, Grafton, & Kilts, 1999; Hamann, Ely, Hoffman, & Kilts, 2002).
Animal studies have also linked physiological activation of the BLA to the modulation of emotional memory. Thus, emotional arousal in animals produces prolonged (hours) increased neuronal firing in the BLA (Pelletier, Likhtik, Filali, & Paré, 2005). Moreover, direct application of corticosterone to the BLA increases the excitability of its principal neurons (Duvarci & Paré, 2007). Direct drug infusion in the BLA can also enhance emotional memory (Fekete et al., 1985; Liang & Lee, 1988; Liang, McGaugh, & Yao, 1990; Roozendaal & McGaugh, 1997; Roozendaal, Schelling, & McGaugh, 2008; Yang et al., 2006).
The enhancement of memory consolidation due to emotional learning and the application of stress hormones to the BLA are dependent on the activation of β-adrenergic receptors within this structure (LaLumiere, Nguyen, & McGaugh, 2004; McGaugh, 1989; Quirarte, Roozendaal, & McGaugh, 1997; Roozendaal, Quirarte, & McGaugh, 2002). Lesions of the BLA or infusions of β-adrenergic receptor antagonists into the BLA block enhancement of memory consolidation (Roozendaal & McGaugh, 1996; Roozendaal, Okuda, Van der Zee, & McGaugh, 2006; Roozendaal et al., 2006). However, the memory enhancing effects of stress, β-adrenergic receptor activation and other modulating treatments are not due to memory enhancement within the BLA itself. For example, lesions of the stria terminalis, efferents of BLA principle neurons, block the enhancement of memory consolidation due to the application of glucocorticoid receptor agonists to the BLA (Roozendaal & McGaugh, 1996; Setlow, Roozendaal, & McGaugh, 2000). Studies have identified the efferent sites of BLA modulatory effects, including the hippocampus, caudate, and cerebral cortex (McGaugh, 2002; Packard, Cahill, & McGaugh, 1994; Roozendaal & McGaugh, 1996).
BLA stimulation enhances cortical activation (Dringenberg, Saber, & Cahill, 2001; Dringenberg & Vanderwolf, 1996). Previous studies have also found evidence for other BLA interactions with cortical function. For example, stimulation of the BLA enhances cortical LTP (Dringenberg, Kuo, & Tomaszek, 2004). However, the means by which the BLA modulates memory in target structures, such as the cortex, are unknown. To address this issue, it would be helpful to identify a memory trace and then observe its modulation by the BLA. Such a candidate for a memory trace is available in the form of CS-specific associatively induced receptive field plasticity in the primary auditory cortex (A1) (Weinberger, 2004).
Classical and instrumental conditioning specifically modify the representations of sounds in A1 that acquire behavioral significance. Such learning causes the frequency receptive fields of cortical neurons to shift toward and to the frequency of the conditioned stimulus (Bakin & Weinberger, 1990; Gao & Suga, 1998; Kisley & Gerstein, 2001). These tuning shifts produce an increase in the number of neurons that become best tuned to the CS frequency, thus increasing the area of representation of the CS frequency. The magnitude of gain in the CS representation is an increasing function of the level of behavioral importance of the CS (Rutkowski & Weinberger, 2005; Weinberger, 2007). Thus, the amount of representational expansion may serve as a memory code for the learned behavioral significance of stimuli (Weinberger, 2001, chap. 16, 2003).
Associative representational plasticity has the major attributes of memory itself and therefore, may constitute a neural memory trace. It is associative (requires pairing), highly specific (responses to frequencies a small fraction of an octave away are attenuated) (Bakin, Lepan, & Weinberger, 1992; Bakin & Weinberger, 1990), discriminative (shifts to a CS+, loses response to a CS–) (Edeline & Weinberger, 1993), develops rapidly (within 5 trials) (Edeline, Pham, & Weinberger, 1993), consolidates (becomes stronger over days without further training) (Galván & Weinberger, 2002) and is enduring (tracked as long as 8 weeks) (Weinberger, Javid, & Lepan, 1993). In toto, these attributes of associative representational plasticity satisfy the criteria for constituting a neural memory trace (Weinberger, 2004).
As the BLA modulates memories, including those stored at least in part in the cerebral cortex, and as associatively induced CS-specific tuning shifts may constitute cortical representations of memory, then the BLA may modulate memories by promoting specific tuning shifts in the cortex. The goal of this study was to conduct an initial test of this hypothesis. A tone was paired with stimulation of the BLA to determine if it is capable of producing CS-specific tuning shifts similar to those that develop during behavioral learning.
2. Methods
2.1. Subjects
Subjects were 21 male Sprague–Dawley rats (320–500 g, Charles River Laboratories, Wilmington, MA) individually housed in a vivarium (maintained at 22 °C, 12/12 h light-dark cycle, on at 7:15 a.m.) with ad libitum access to food and water. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines.
2.2. Surgical procedures and electrophysiology
The entirety of the experiment was conducted with the rats under general anesthesia (urethane, 1.6 g/kg, i.p.). Bronchial secretions were minimized by treatment with atropine sulfate (0.25 mg/kg, i.m.) and body temperature was maintained at 37 °C with a homeothermic heating blanket (Harvard apparatus, Kent, England). Subjects were secured to a stereotaxic frame within a sound attenuated chamber (IAC, New York, NY) via a pedestal created with dental acrylic (Teets denture material, co-Oral-Ite Dental Mfg. Co., Diamond Springs, CA) such that the ears were not obstructed by earbars, yet the subjects’ head remained within the stereotaxic plane. A craniotomy over the right primary auditory cortex allowed for multiunit extracellular as well as EEG recordings with a linear four-electrode array (305–505 μm separation) of Parylene-coated tungsten microelectrodes (0.2–3.0 MΩ, FHC), in layers IV–V of primary auditory cortex.
Neural activity was amplified (1000×), bandpass filtered (0.3–3.0 kHz, Tucker–Davis technologies [TDT, Alachua, FL] RA16 Medusa Base Station) and monitored via an oscilloscope (Tektronix 5111) and loudspeaker system (Grass AM8). The electroencephalogram (EEG) was monitored with the same cortical electrodes, bandpass filtered at 10–100 Hz (TDT RA16 Medusa Base Station) and monitored through Matlab custom software. A calibrated speaker (Aiwa) was placed at the entrance to the ear canal contralateral to the craniotomy. Acoustic stimuli were generated using TDT hardware and software. First, white noise stimuli (1.0–50.0 kHz) were presented every 700 ms (0–80 dB SPL in 10 dB SPL steps) 20 times, to determine threshold response. Then, pure tone bursts (50 ms duration, cosine-squared gate, rise/fall time of 10 ms) of 0.5–54.0 kHz were presented every 700 ms from 0 to 80 dB SPL in 10 dB steps (252 stimuli combinations) and repeated 5 times pseudo-randomly to determine frequency response.
A second craniotomy was then created so that a concentric bipolar electrode (stainless steel CBJPM60 outer diameter of 550 μm, inner diameter 250 μm, epoxylite-coated; FHC, Bowdoin, ME) could be placed within the ipsilateral BLA (coordinates: A–P –2.76; M–L 5.0; Paxinos & Watson, 2007). The final dorsal–ventral coordinate was determined using bi-polar electrical stimulation (Grass S8800 stimulator and Stimulation Isolation Unit) to confirm placement within the BLA (400 ms train, 0.2 ms opposite polarity, 1.0 ms inter-pulse interval, delivered at 100 Hz, 400 ± 16.54 μA). BLA placement was assumed when stimulation produced 3–10 s desynchronization of the cortical EEG (Dringenberg & Vanderwolf, 1996). Some animals failed to exhibit EEG desynchronization; these later proved to have electrode placements outside of the BLA (see Section 3).
2.3. Training protocol
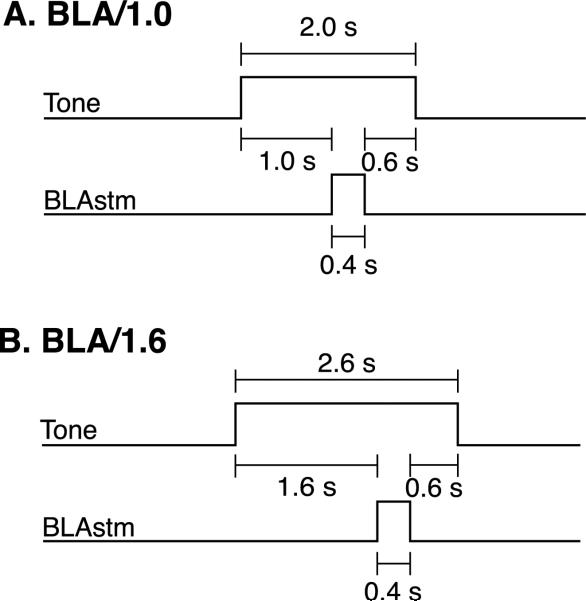
Two groups of rats received 100 trials of a pure tone, which was always paired with a 400 ms stimulation train of the BLA (BLAstm). Intertrial intervals averaged 30 s (20–40 s). The stimulation parameters within a subject were always the same as those that produced EEG desynchronization during electrode placement. The BLA/1.0 group (n = 16) received a 2 s tone presentation paired with a 400 ms BLAstm that occurred 1 s after tone onset, and the tone continued for 600 ms after the offset of BLAstm (Fig. 1A). This protocol was used to simulate contextual fear conditioning, where the context (tone) is present both before and after the offset of a fear inducing experience. The BLA/1.6 group (n = 5) received a 2.6 s tone and a 400 ms BLAstm that occurred 1.6 s after tone onset and the tone continued for 600 ms post-BLA stimulation (Fig. 1B). This group was used to determine if the precise tone–BLAstm interval was relevant.
Fig. 1.
(A) BLA/1.0 training protocol consisted of at 2.0 s tone paired with 0.40 s BLA stimulation train. The tone–BLA stimulation interval was 1.0 s. The tone then continued for 0.60 s after BLA stimulation. (B) BLA/1.6 training protocol consisted of a 2.6 s tone paired with a 0.40 s BLA stimulation train. The tone–BLA stimulation interval was 1.6 s, the tone then continued for 0.60 s after BLA stimulation.
2.4. Neural data analysis
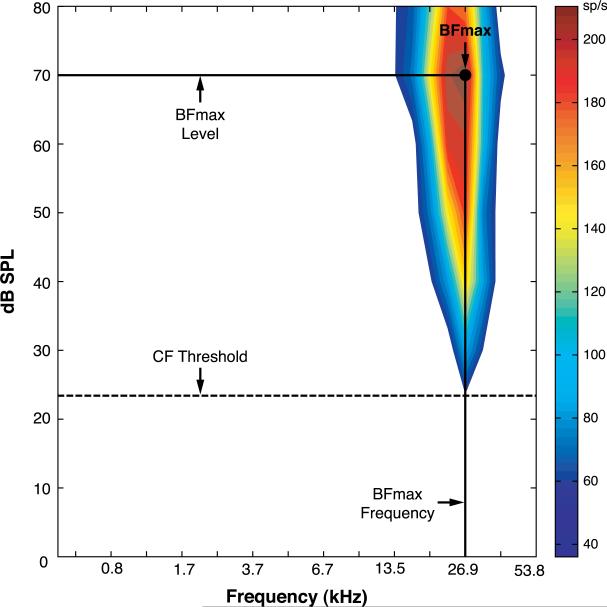
A1 was defined by a general progression of low to high characteristic frequencies along the posterior to anterior axis. Anterior placements that resulted in a reversal in the CF progression were defined as lying in the anterior auditory field and were excluded from analyses (Rutkowski, Miasnikov, & Weinberger, 2003). Further, the posterodorsal, dorsal and anterodorsal belt areas were identified as more responsive to noise than to tone and these areas were considered to be outside of A1 and also were excluded (Rutkowski et al., 2003). Frequency response areas (FRA) were created offline (Matlab custom software) using evoked spike-time data, 6–40 ms following tone onset. Baseline recordings were taken during a 0–50 ms time window before tone presentation. Evoked activity was defined as activity ≥2.5 SE above mean spontaneous baseline activity. The characteristic frequency (CF) was the frequency that elicited the lowest threshold evoked discharge. If more than one stimulus frequency elicited the threshold response, then the frequency that elicited the greatest number of spikes was identified as the CF. The maximum best frequency (BFmax), i.e., frequency and level combination that produced the greatest evoked discharge within the FRA, was used for all analyses (Fig. 2).
Fig. 2.
An example of a frequency response area (FRA) obtained before training. The BFmax is defined as the frequency–intensity (dB level) that elicited the greatest neuronal discharge. In this illustration, the BFmax was 26.9 kHz at 70 dB SPL. The BFmax was used for all analyses.
To confirm stability of A1 tuning, at least two FRAs were obtained before training. A pure tone that did not elicit the best response (0.5–5.0 octaves from the BFmax; mean = 1.92 ± 0.25; median = 1.75) was chosen as the auditory stimulus to-be-paired with BLA stimulation, to enable the detection of treatment-induced tuning shifts (for convenience, this tone is referred to as the “CS”, without implying a strict Pavlovian protocol; see below). Post-training FRAs were obtained immediately, 45 min and 75 min after training and were analyzed for the development of any A1 tuning shifts across time. The post-training frequency responses were then compared to the pre-training responses to determine if a tuning shift had occurred. A shift index (SI) was used to quantify the extent and direction of shift, as follows:
A positive SI indicates a shift towards the CS, while a negative SI indicates a shift away from the CS. A complete shift to the CS frequency after training would produce an SI = 1.0. An SI was calculated for each FRA obtained after training. Electrodes with pre-training recordings that were not stable (i.e., failed to maintain the same CF and BFmax) were excluded from all analyses. Quantification of the stability of the pre-training tuning was accomplished by calculating a pre-training “SI” score (SI = Pre 2 BFmax – Pre 1 BFmax/CS – Pre 1 BFmax).
2.5. Histology
Rats were sacrificed (Euthasol, 50 mg/kg, i.p.) and perfused with 0.9% saline, followed by 10% formalin. Brains were removed and placed into 20% sucrose solution until sectioned with a microtome. Brains were frozen and sliced into 50 μm slices, mounted on gelatinized slides and stained with thionine for visualization of BLA electrode placements. Placements were determined under light microscope with the assistance of the Paxinos and Watson rat brain atlas (Paxinos & Watson, 2007).
3. Results
3.1. Location of stimulating electrodes
Fig. 3 summarizes the placements of all stimulating electrodes. Of the 16 animals that were trained in the BLA/1.0 protocol, 11 had electrode placements within the BLA. The five animals with placements outside of the BLA failed to exhibit EEG desynchronization and served as a nonBLA stimulation control group, referred to hereafter as the nonBLA/1.0 group. All five of the animals trained in the BLA/1.6 protocol had placements within the BLA.
Fig. 3.
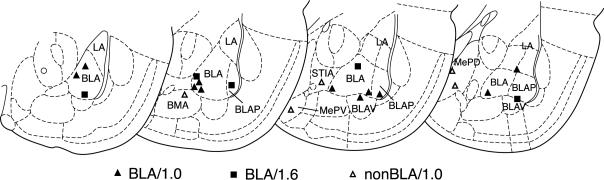
Electrode placements for each animal for each group. BLA/1.0, n = 11; BLA/1.6, n = 5; nonBLA/1.0, n = 5. Basal lateral amygdala (BLA); posterior basal lateral amygdala (BLAP); ventral basal lateral amygdala (BLAV); lateral amygdala (LA); basomedial amygdala (BMA); postereodorsal medial amygdala (MEPD); postereoventral medial amygdala (MEPV); bed nucleus of the stria terminalis (STIA).
3.2. Effects of BLA stimulation on frequency tuning
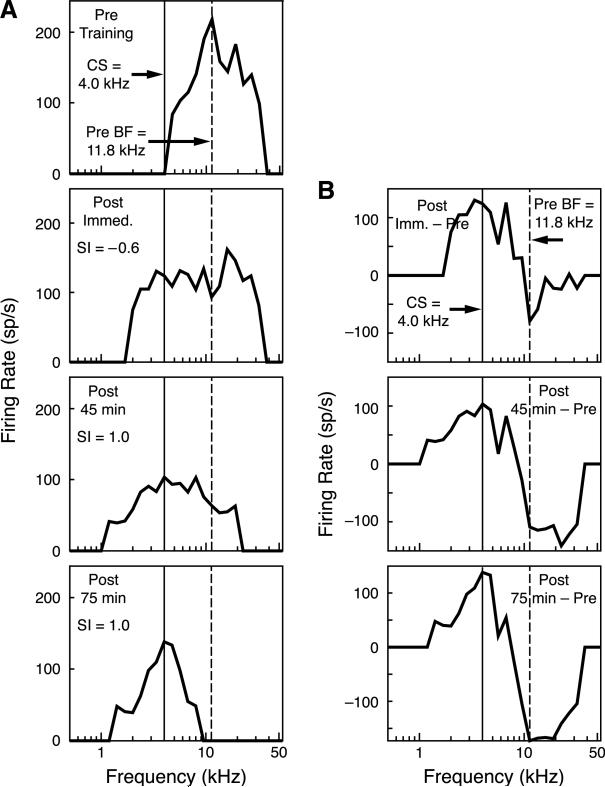
Stimulation of the BLA did induce frequency tuning shifts. Fig. 4A provides an example of the type of shift induced in the BLA/1.0 group over the 75 min of post-training recording. The pre-training BFmax was 11.3 kHz, the frequency chosen for the CS was 4.0 kHz which was slightly beyond the frequency receptive field. Immediately after training, the BFmax exhibited a slight shift away from the CS, resulting in an SI score of –0.06. However, the tuning curve became broader and now extended down to and beyond the CS frequency. Also, the shift away from the CS was a result of the loss of responsiveness at the BFmax. With the passage of time, further development was evident. By 45 min after training, the BFmax had shifted to the CS frequency, resulting in an SI score of 1.0. Also, responses to the higher frequencies were diminished. At 75 min after training, the BFmax remained at the CS frequency, the cells no longer responded to the pre-training BFmax, and the frequency receptive field became more sharply tuned around the CS frequency. Fig. 4B shows the differences between post-training and pre-training tuning. Immediately after training, the greatest loss of response was at the pre-training BFmax, while conversely, the greatest increase in response was to the CS frequency. The tuning shift continued to develop in the absence of further training. This pattern became stronger by 45 min post-training and was accentuated at 75 min post-training.
Fig. 4.
(A) Example of tuning curves recorded from an animal in the BLA/1.0 group. Top row is the pre-training tuning curve with a BFmax that was at 11.3 kHz; the training frequency (CS) selected was 4.0 kHz. Immediately after training, there was a large decrease in responsiveness at the BFmax and an increase in responsiveness at the CS frequency. Tuning shifted to the CS frequency at 45 min (SI = 1.0) and the receptive field became even more sharply tuned at 75 min, while maintaining its peak at the CS frequency (SI = 1.0). (B) Differences between the pre- and post-training tuning curves. Immediately after training, there was a maximum decrease in responsiveness to the BFmax and a maximum increase in response to the CS frequency. This pattern continued and grew 45 min after training and increased even more at the 75-min time period.
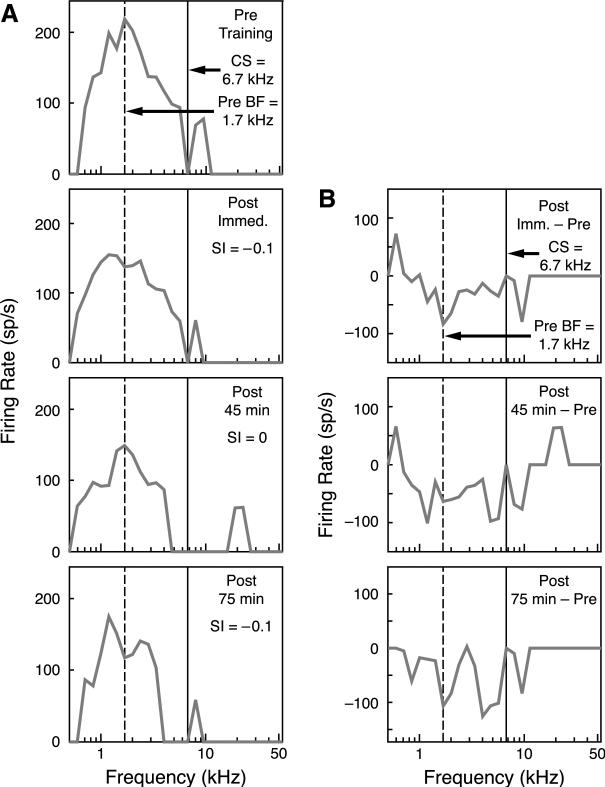
In contrast, the BLA/1.6 group did not develop CS-specific tuning shifts. Fig. 5 presents an example. Although the CS (6.7 kHz) was at the edge of the frequency RF, similar to the example of the BLA/1.0 animal in Fig. 4, there was no comparable tuning shift. The SI values were –0.1, 0.0 and 0.1 immediately, 45 min and 75 min after training, respectively (Fig. 5A). Similarly, the differences in tuning between the post-training period and the pre-training period exhibited no pattern of specific decrease at the pre-training BFmax or specific increase at the CS frequency (Fig. 5B).
Fig. 5.
(A) Examples of tuning curves recorded from an animal in the BLA/1.6 group. Tuning Training shifts were negligible in this group. The top row is the pre-training tuning curve with a BFmax at 1.7 kHz; the chosen was 6.7 kHz. Immediately after training, there was a slight decrease in firing rate at the BFmax and a slight shift in the BFmax frequency away from the training frequency. At 45 min after training, the BFmax frequency returned to the pre-training BFmax (1.7 kHz) and the firing rate did not change. At 75 min after training, the there was a slight shift away from the training frequency again and the firing rate did not change. (B) Differences between the pre- and post-training tuning curves. At each time period, there was no clear shift in tuning away from or towards the training frequency.
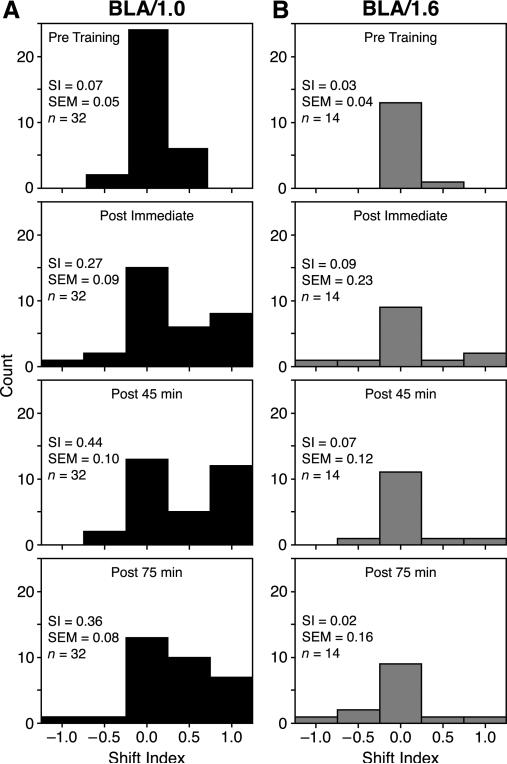
Fig. 6 presents the distribution of SI scores of both the BLA/1.0 and the BLA/1.6 groups at each time period. The BLA/1.0 mean SI score for the pre-training tuning was 0.07 (±0.05) indicating tuning stability before training. However, immediately after training, the mean SI of this group rose to 0.27 (±0.09), showing a substantial CS-directed tuning shift; however this shift was not statistically significant from baseline (Wilcoxen signed-ranks, z = 1.62, p = .11). The mean SI score increased at 45 min post-training, to 0.44 (±0.10), revealing a characteristic of neural consolidation, i.e., continued increase in strength in the absence of further training which was significant from baseline (z = 2.94, p = .003). Further, the 45 min post-training time point was also significantly greater than the immediate post-training time point (z = 2.27, p = .023). This marked shift was retained at the 75 min retention interval. Although it was somewhat reduced, i.e., SI = 0.36 (±0.08), the shift was significantly greater than baseline (z = 2.76, p = .006) (Fig. 6A), and there was no difference between the 45 and 75 min retention intervals (z = 0.11, p = .91).
Fig. 6.
The distribution of SI scores across the pre-training and post-training periods. (A) BLA/1.0 tuning. Note the increase in positive SI scores indicating tuning shifts toward the CS frequency. (B) BLA/1.6 tuning. Note the lack of CS-directed tuning shifts for any post-training time period. Bin-width of distributions = 0.5 SI; zero bin range = –0.25 to 0.25 SI.
The BLA/1.6 mean SI score for pre-training tuning was 0.03 (±0.04), also indicating frequency stability before training. However, BLAstm did not produce substantial tuning shifts in this group. Immediately after training the mean SI was 0.09 (±0.23) (z = 1.29, p = .20); 45 min later it was 0.07 (±0.12) (z = 1.50, p = .13). At 75 min after training, the SI was 0.02 (±0.16) indicating a slight shift away from the CS, which was not significant from baseline (z = 1.08, p = .28) (Fig. 6B).
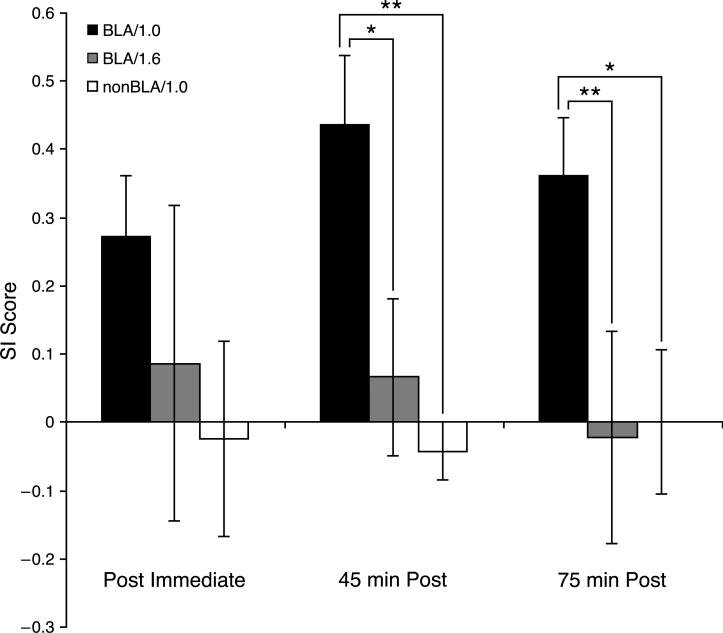
The groups did not differ in pre-training SI values, (z = 0.83, p = .41, Fig. 7) or in SI values immediately after training (z = 1.93, p = .054). However, the BLA/1.0 group had significantly greater tuning shifts thereafter: 45 min post-training, (z = 2.28, p = .023); 75 min post-training, (z = 2.71, p = .007, Fig. 7). In summary, the BLA/1.0 group showed a shift towards the CS that continued to develop overtime but the BLA/1.6 group did not.
Fig. 7.
SI scores (±SE) for all three groups, across time. The BLA/1.0 group had a significantly larger SI score than the BLA/1.6 and the nonBLA/1.0 groups at 45 and 75 min after training. *p < .05; **p < .01.
3.3. BLA specificity
To determine if stimulation of the BLA was necessary for tuning shifts, we examined the nonBLA/1.0 group, which was trained in the same protocol but had placements outside of the BLA (Fig. 3). Fig. 7 summarizes the SI scores across time of both groups, plus the BLA/1.6 group. The pre-training SI score of the nonBLA/1.0 group was 0.02 (±0.04) indicating tuning stability before training and was not significantly different from the BLA/1.0 group (z = 0.71, p = .48). Immediately after training, the nonBLA/1.0 group showed essentially no shift (SI = 0.02 ± 0.14). Although the BLA/1.0 group exhibited a substantial tuning shift (SI = 0.27 ± 0.09), as noted previously, this proved not to be statistically different from the nonBLA/1.0 group (z = 1.60, p = .11). However, the BLA/1.0 group had significantly greater shifts at 45 min (z = 2.85, p = .004) and 75 min (z = 2.52, p = .012). Moreover, the nonBLA/1.0 group never exhibited any change from baseline at any post time period (Wilcoxen signed-ranks; post immediate, z = 0.91, p = .36; 45-min post, z = 0.80, p = .42; 75-min post, z = 0.31, p = .75). Therefore, tuning shifts were found only for stimulation of the BLA itself.
3.4. Other potential factors
Although the differences in training protocol between the BLA/1.0 and BLA/1.6 groups (e.g., CS–BLAstm interval of 1.0 vs. 1.6 s) appear to be responsible for the differences in representational plasticity, other factors might have been involved (Table 1A). If some frequencies are more “plastic” than others are, then differences in the pre-training BFmax frequencies might have affected the results. However, the groups did not differ significantly (z = 0.866, p = .387). Another potential factor is the octave distance between the pre-training BFmax and the CS frequency. For example, if the CS frequency were closer to the BFmax in the BLA/1.0 group, then it might have been easier to induce a tuning shift than in the BLA/1.6 group. However, there was no significant difference in the octave distance (z = 0.13, p = .90). If the level of BLAstm current were higher in the BLA/1.0 group that could explain the group differences in tuning shifts. However, the intensity of stimulation was not different between groups (z = 0.61, p = .55).
Table 1.
Comparison of groups BLA/1.0 and BLA/1.6 on predisposing factors and magnitude of evoked discharges.
| Group | BFmax frequency (kHz) | CS–BF Octave distance (octaves) | BLA stimulationcurrent (μA) |
|---|---|---|---|
| (A) Predisposing factors | |||
| BLA/1.0 | 22.20 (±2.76) | 1.90 (±0.25) | 359.38 (±19.99) |
| BLA/1.6 | 27.60 (±4.54) | 1.80 (±0.18) | 365.71 (±26.47) |
| Z-score (Mann–Whitney) | 0.87 | 0.13 | 0.61 |
| Responses to BFmax (spikes/s) |
||||
|---|---|---|---|---|
| Pre | Imm | 45 min | 75 min | |
| (B) Evoked discharges | ||||
| BLA/1.0 | 220.60 (±11.47) | 205.64 (±11.85) | 204.04 (±12.45) | 195.58 (±14.84) |
| BLA/1.6 | 181.40 (±12.86) | 194.34 (±14.11) | 183.69 (±13.57) | 182.36 (±14.39) |
| Z-score Mann–Whitney) | 1.6 | 0.53 | 0.24 | 0.63 |
Mean ± SE.
The level of responsivity might have been a factor. To assess this possibility the evoked firing rates were analyzed within and between both groups. The BLA/1.0 mean firing rate did not change significantly throughout the recording session (Friedman χ2 = 2.48, p = .48). Similarly, there were no significant differences in the firing rate of BLA/1.6 across time (χ2 = 1.63, p = .65). Finally, firing rates between groups were evaluated for each time period and were not significantly different (Table 1B).
BLA/1.0 and nonBLA/1.0 groups were also compared on several factors other than differential electrode placements. There were no significant differences between groups for BFmax frequency or CS–BF octave distance (Table 2A). Stimulation current was significantly higher in the nonBLA/1.0 (z = 3.91, p = .0005) because they did not exhibit EEG desynchronization during electrode placement (see Section 2), although attempts were made to elicit this effect at higher currents. However, a higher current level cannot account for failure to obtain tuning shifts. A comparison of evoked firing rates revealed no significant differences immediately or 45 min after training, but a higher rate for the nonBLA/1.0 group at 75 min after training (Table 2B). A higher rate of response shows that the absence of tuning shifts is not due to a lack of responsivity. In toto, the evidence indicates that stimulation of the BLA along with the BLA/1.0 training paradigm is necessary to produce large and enduring CS-specific tuning shifts.
Table 2.
Comparison of groups BLA/1.0 and nonBLA/1.0 on predisposing factors and magnitude of evoked discharges.
| Group | BFmax frequency (kHz) | CS–BF octavedistance (octaves) | BLA stimulationcurrent (μA) |
|---|---|---|---|
| (A) Predisposing factors | |||
| BLA/1.0 | 22.20 (±2.76) | 1.90 (±0.25) | 359.38 (±19.99) |
| NonBLA/1.0 | 26.90 (±3.95) | 1.20 (±0.17) | 527.14 (±28.64) |
| Z-score Mann–Whitney) | 0.99 | 1.47 | 3.91*** |
| Responses to BFmax (spikes/s) |
||||
|---|---|---|---|---|
| Pre | Imm | 45 min | 75 min | |
| (B) Evoked discharge | ||||
| BLA/1.0 | 220.60 (±11.47) | 205.64 (±11.85) | 204.04 (±12.45) | 195.58 (±14.84) |
| NonBLA/1.0 | 232.90 (±21.34) | 216.40 (±18.98) | 208.50 (±15.23) | 272.40 (±15.88) |
| Z-score (Mann–Whitney) | 0.69 | 0.62 | 0.17 | 2.77** |
Mean ± SE.
p <.01.
p <.001.
4. Discussion
4.1. Summary and validity of findings
The results demonstrate that electrical stimulation of the BLA is capable of producing shifts of frequency tuning in the primary auditory cortex. Moreover, this receptive field plasticity is directed toward the tonal frequency with which it was paired (the “CS”). Furthermore, these induced specific tuning shifts are long-lasting (retained for 75 min, the longest period tested) and their magnitude increases over time in the absence of further pairing (i.e., they consolidate). These attributes of receptive field plasticity are the same as those that develop during natural learning (e.g., Bakin & Weinberger, 1990; Edeline & Weinberger, 1993; Galván & Weinberger, 2002).
However, the conclusion that the BLA can shift tuning depends upon excluding all alternative explanations of the findings. We examined the potential effects of a large number of other factors, including BFmax frequency, the octave distance from CS to BFmax frequency, stimulation current and rate of evoked discharges (Tables 1 and 2). None of them could account for the findings. In addition, stimulation of the BLA was found to be necessary as stimulation of sites near, but outside of the BLA, was ineffective (Table 2).
4.2. Apparent unusual nature of the findings
The findings may be viewed as strange, depending upon one's point of view, for several reasons. First, tuning shifts occurred when the tone preceded BLAstm by 1.0 s but not by 1.6 s, indicating a high degree of temporal specificity. Second, the use of tone–BLA “forward pairing”, suggests a classical conditioning protocol, yet there was no “control” group for non-associative factors. Third, the presentation of tone continued after the offset of BLA stimulation, which does not conform to any standard conditioning protocol. It will prove helpful to initiate the discussion of these in reverse order.
4.2.1. The overlap protocol
The continued presentation of the tone after offset of BLA stimulation (“overlap” protocol; Fig. 1) was designed as a simulacrum of experience-dependent release of stress hormones and subsequent activation of the BLA. This protocol might seem inappropriate because ordinarily there is a temporal delay between an experience and the ultimate activation of the BLA (Hatfield & McGaugh, 1999; LaLumiere, Buen, & McGaugh, 2003; Roozendaal, Castello, et al., 2008). One might have expected the tone to be followed by a period of silence, and then BLA stimulation, as that might be a closer approximation of the natural situation. However, one must bear in mind that this initial study used animals that were under deep (i.e., surgical level) general anesthesia. Under such a state, many normal processes are affected. For example, the memory trace of a tone is less robust and shorter-lasting in a cortex that is in a depressed state. Therefore, had the BLA stimulation been given in a standard delay or trace classical conditioning protocol, a failure to obtain specific frequency plasticity would have been uninterpretable because it might have been due to a weak auditory memory trace rather than an inability of the BLA to specifically modulate the auditory cortex. Thus, the “overlap” protocol was designed to provide BLA stimulation effects while the auditory cortex still processed the tone.
4.2.2. Associativity
The forward pairing of a CS and US (in this case a tone with BLA stimulation) is a cardinal feature of Pavlovian conditioning. Therefore, it might be assumed that we should have included a non-associative control group. This assumes that the goal was to determine if BLAstm effects are associative. Moreover, this assumption might be supported by the widespread belief that the amygdala is the site of convergence of a tone and shock underlying auditory fear conditioning. However, the evidence upon which this claim ultimately rests is the demonstration of conditioning-related plasticity in the amygdala (Fanselow & LeDoux, 1999) because lesion data are not decisive. As the amygdala has some function in fear conditioning, the existence of plasticity per se is neither in dispute nor decisive. There are several reasons for concluding that the claims of an amygdala locus of memory are premature (Berlau & McGaugh, 2003; Cahill, Weinberger, Roozendaal, & McGaugh, 1999; Vazdarjanova & McGaugh, 1998). Among these is the fact that afferent to the amygdala is the magnocellular medial geniculate nucleus of the auditory thalamus, in which tone–shock convergence and the rapid development of plasticity in auditory fear conditioning have been documented in several species in no less than 25 papers from many laboratories (reviewed in Weinberger, 2008). In contrast, the role of the BLA in the modulation of memory is well established (McGaugh, 2004).
In short, the current study is not an attempt to use BLA stimulation as a substitute for a shock unconditioned stimulus. Hence, it does not involve the issue of associativity. Rather, this study is a test of the hypothesis that the BLA can modulate the representation of specific sensory representations elsewhere in the brain. The characteristics of associative receptive field plasticity in the primary auditory cortex satisfy the criteria for the representation of specific stored information. As noted above (Section 1), these attributes transcend associativity and notably include the three attributes of BLA-induced RF plasticity discovered in the present study: (a) a high degree of specificity, (b) increased strength over time (i.e., “consolidation”) and (c) retention. Thus, the present findings support this hypothesis of BLA modulation of sensory memory representations.
4.2.3. Temporal constraints
After having obtained tuning shifts in the BLA/1.0 group, we investigated the temporal dimension with the BLA/1.6 group. We found that BLA stimulation can produce an enduring shift of tuning (i.e., for at least 75 min) if it is presented 1.0 s after tone onset but no tuning shift if presented 1.6 s after tone onset. We focused on the latency between tone onset and BLAstm onset because the durations of BLAstm and the post-BLA tone period of 0.6 s were identical in both the BLA/1.0 and the BLA/1.6 groups (Fig. 1). This degree of temporal specificity was unexpected because the temporal relationships between an experience and the effects of the release of stress hormones and the subsequent engagement of neurotransmitter systems in the BLA is not so temporally constrained (McIntyre, Hatfield, & McGaugh, 2002; Quirarte, Galvez, Roozendaal, & McGaugh, 1998). We have no definitive explanation for this finding but note the following. First, the deeply anesthetized preparation, while useful, is far from an adequate substitute for the waking, behaving and learning animal. Thus, one or more characteristics of the anesthetized state may be responsible. There is increasing evidence that the auditory cortex of anesthetized subjects responds strongly to the onset of tones but not to their continued presence. For example, the primary auditory cortex is especially sensitive to acoustic onset transients (Heil & Irvine, 1998; Phillips, Hall, & Boehnke, 2002) and indeed specific auditory cortical plasticity may be dependent upon attention to onset transients (Berlau & Weinberger, 2008). The delay between the onset of the tone and the onset of BLAstm was longer in the group for which BLAstm failed to shift tuning. It is possible that the neural effects of the tone onset in the primary auditory cortex had faded too much by 1.6 s after tone onset, such that the BLAstm could not modulate a weak acoustic representation. In general support of this possibility, the duration of the human auditory memory trace is briefest in the primary auditory cortex compared to other, “higher” auditory association fields (Lü, Williamson, & Kaufman, 1992). Future studies of BLA modulation should be conducted in the unanesthetized, waking animal.
4.3. Possible mechanisms of BLA modulation of the auditory cortex
The present findings were predicted in general by a model of learning-induced specific receptive field plasticity (Weinberger, 1995; Weinberger, 2008; Weinberger et al., 1990). This model made no distinction of involved nuclei with the amygdala, but postulated that the amygdala, having received specific enhanced input from the magnocellular medial geniculate nucleus, would activate the cholinergic nucleus basalis (NB) to release acetylcholine in the auditory cortex, and thereby greatly promote the long-term storage of information. Since the formation of this model in 1990, several recent findings have proven to be highly relevant.
Stimulation of the BLA produces not only specific tuning shifts in the primary auditory cortex but also EEG activation of the cerebral cortex. This effect on cortical state apparently is mediated via the cholinergic nucleus basalis (NB) (Dringenberg & Vanderwolf, 1997). Stimulation of the nucleus basalis also produces EEG activation (e.g., Buzsaki et al., 1988) and tone paired with NB stimulation produces CS-directed tuning shifts (Bakin & Weinberger, 1996; Bjordahl, Dimyan, & Weinberger, 1998; Dimyan & Weinberger, 1999; Ma & Suga, 2003). These findings are consistent with the possibility that BLA modulation of frequency receptive fields in the auditory cortex is also mediated in part via the nucleus basalis, particularly as the BLA projects to the NB in the rat (Grove, 1988; see also Russchen, Amaral, & Price, 1985).
While the present findings demonstrate the ability of the BLA to specifically modulate representations of sensory memory, this initial study was not intended to link BLA stimulation to actual behavioral memory via CS-directed plasticity. However, in light of previous findings, the present results are consistent with a particular systems-level mechanism for memory modulation.
First, tone paired with stimulation of the NB not only induces specific tuning plasticity, but also induces actual behavioral memory (e.g., McLin, Miasnikov, & Weinberger, 2002; Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008; Miasnikov, Chen, & Weinberger, 2006; Miasnikov, Chen, & Weinberger, 2008; Weinberger, Miasnikov, & Chen, 2006). Second, tone paired with NB stimulation also increases the number of neurons that become best tuned to the paired frequency (Kilgard & Merzenich, 1998). Third, the number of neurons that become best tuned to a paired frequency increases directly as a function of the degree of the tone's behavioral meaning or importance (Rutkowski & Weinberger, 2005). The latter finding suggests that the brain has a memory code for the acquired behavioral significance of environmental events, specifically the number of neurons that become tuned to that event or stimulus (Weinberger, 2001, chap. 16).
Integrating these findings suggests that the BLA may strengthen memory, in part, by shifting tuning to sensory memory representations of important events. As hypothesized in the model, the BLA may well be a critical structure that drives the nucleus basalis to release acetylcholine into the cerebral cortex. This is consistent with the recent finding that lesions of the NB block the memory enhancing effect of norepinephrine injected into the BLA (Power, Thal, & McGaugh, 2002). However, while ACh and the NB may be key components in BLA modulation of memory, the roles of other transmitter systems need to be considered as well. For example, nor-adrenergic, dopaminergic and serotonergic axons engage cholinergic cells in the NB (Smiley, Subramanian, & Mesulam, 1999) and norepinephrine excites these neurons (Fort, Khateb, Pegna, Mühlethaler, & Jones, 1995). Also, histamine applied to the NB facilitates cortical activation (Dringenberg & Kuo, 2003) and increases the release of ACh in the cortex (Cecchi, Passani, Bacciottini, Mannaioni, & Blandina, 2001). Stimulation of the ventral tegmental area (VTA) can also increase the representation of a tone with which it is presented, perhaps by the release of dopamine (Bao, Chan, & Merzenich, 2001). It will now be appropriate both to extend the current study to the waking, behaving animal and to forge a tighter link among the BLA, neurotransmitter systems, modulation of the auditory cortex and behavioral memory.
Acknowledgments
We thank Drew B. Headley, Gabriel K. Hui and Jacquie Weinberger for assistance. This study was funded by the NIDCD Grant #DC-05592 (N.M.W.), NIMH Grant #MH-12526 (J.L.M.) and the APA/DPN fellowship #5-T32-MH-18882 (C.M.C.).
References
- Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Research. 1992;577(2):226–235. doi: 10.1016/0006-8993(92)90278-h. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research. 1990;536(1–2):271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Rojas Y, Capdevila V, González O, Almaguer-Melian W. Stimulation of the basolateral amygdala improves the acquisition of a motor skill. Restorative Neurology and Neuroscience. 2006;24(2):115–121. [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Basolateral amygdala lesions do not prevent memory of context-footshock training. Learning & Memory. 2003;10(6):495–502. doi: 10.1101/lm.64003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau KM, Weinberger NM. Learning strategy determines auditory cortical plasticity. Neurobiology of Learning and Memory. 2008;89(2):153–166. doi: 10.1016/j.nlm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18(2):379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. Journal of Neuroscience. 1988;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23(2):227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(19):RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Passani MB, Bacciottini L, Mannaioni PF, Blandina P. Cortical acetylcholine release elicited by stimulation of histamine H1 receptors in the nucleus basalis magnocellularis: A dual-probe microdialysis study in the freely moving rat. European Journal of Neuroscience. 2001;13(1):68–78. [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1999;113(4):691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Kuo MC. Histaminergic facilitation of electrocorticographic activation: Role of basal forebrain, thalamus, and neocortex. European Journal of Neuroscience. 2003;18(8):2285–2291. doi: 10.1046/j.1460-9568.2003.02975.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Kuo MC, Tomaszek S. Stabilization of thalamo-cortical long-term potentiation by the amygdala: Cholinergic and transcription-dependent mechanisms. European Journal of Neuroscience. 2004;20(2):557–565. doi: 10.1111/j.1460-9568.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Saber AJ, Cahill L. Enhanced frontal cortex activation in rats by convergent amygdaloid and noxious sensory signals. Neuroreport. 2001;12(11):2395–2398. doi: 10.1097/00001756-200108080-00022. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: An amygdaloid activating system. Experimental Brain Research. 1996;108(2):285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Neocortical activation: Modulation by multiple pathways acting on central cholinergic and serotonergic systems. Experimental Brain Research. 1997;116(1):160–174. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Paré D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. Journal of Neuroscience. 2007;27(16):4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline J-M, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1993;107(4):539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: Selective retuning independent of task difficulty. Behavioral Neuroscience. 1993;107(1):82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fekete M, Balázs M, Telegdy G, Schally AV. Effects of intracerebroventricular administration of ovine corticotropin-releasing factor (CRF 1–41) on passive avoidance behaviour: Lack of influence on monoamine contents of limbic brain areas. Neuropeptides. 1985;6(4):283–292. doi: 10.1016/0143-4179(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Pegna A, Mühlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. European Journal of Neuroscience. 1995;7(7):1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS. Effect of phentolamine administration into the amygdala complex of rats on time-dependent memory processes. Behavioral and Neural Biology. 1981;31(1):90–95. doi: 10.1016/s0163-1047(81)91130-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: Evidence for a specific neurochemical system in the amygdala. Science. 1977;198(4315):423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiology of Learning and Memory. 2002;77(1):78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: Effect on memory varies with footshock level. Brain Research. 1975;86(3):509–513. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behavioral Biology. 1975;13(2):145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB, McGaugh JL. Effects of hormones on time-dependent memory storage processes. Progress in Brain Research. 1975;42:210–211. doi: 10.1016/s0079-6123(08)63665-1. [DOI] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: Afferent connections. Journal of Comparative Neurology. 1988;277(3):315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory. 1999;71(2):232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Heil P, Irvine DR. Functional specialization in auditory cortex: Responses to frequency-modulated stimuli in the cat's posterior auditory field. Journal of Neurophysiology. 1998;79(6):3041–3059. doi: 10.1152/jn.1998.79.6.3041. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning and Memory. 2004;81(1):67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hui IR, Hui GK, Roozendaal B, McGaugh JL, Weinberger NM. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiology of Learning and Memory. 2006;86(2):160–163. doi: 10.1016/j.nlm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Nagahara AH, McGaugh JL. Memory enhancement with intra-amygdala post-training naloxone is blocked by concurrent administration of propranolol. Brain Research. 1989;476(1):94–101. doi: 10.1016/0006-8993(89)91540-0. [DOI] [PubMed] [Google Scholar]
- Kety SS. The possible role of the adrenergic systems of the cortex in learning. Research Publications: Association for Research in Nervous and Mental Disease. 1972;50:376–389. [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. Journal of Neuroscience. 2001;21(14):5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. European Journal of Neuroscience. 2001;13(10):1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S, Tarte RD. The relationship of arousal to short- and long-term verbal recall. Canadian Journal of Psychology. 1963;17(4):393–397. doi: 10.1037/h0083278. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience. 2003;23(17):6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: Involvement of noradrenergic and cholinergic systems. European Journal of Neuroscience. 2004;20(10):2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Research. 1986;368(1):125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang KC, Lee EH. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology. 1988;96(2):232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Research. 1990;508(2):225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Lü ZL, Williamson SJ, Kaufman L. Human auditory primary and association cortex have differing lifetimes for activation traces. Brain Research. 1992;572(1–2):236–241. doi: 10.1016/0006-8993(92)90475-o. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. Journal of Neurophysiology. 2003;89(1):90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- McCarty R, Gold PE. Plasma catecholamines: Effects of footshock level and hormonal modulators of memory storage. Hormones and Behavior. 1981;15(2):168–182. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annual Review of Neuroscience. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Archer T, Ross SB. Evidence for a role of the locus coeruleus noradrenaline system in learning. Neuroscience Letters. 1980;20(3):351–356. doi: 10.1016/0304-3940(80)90173-1. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(18):8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: Implications for facilitated consolidation of emotional memories. Learning & Memory. 2005;12(2):96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Hall SE, Boehnke SE. Central auditory onset responses, and temporal asymmetries in auditory perception. Hearing Research. 2002;167(1–2):192–205. doi: 10.1016/s0378-5955(02)00393-3. [DOI] [PubMed] [Google Scholar]
- Power AE, Thal LJ, McGaugh JL. Lesions of the nucleus basalis magnocellularis induced by 192 IgG–saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2315–2319. doi: 10.1073/pnas.022627799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research. 1998;808(2):134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF, Crow TJ. A new approach to the role of noradrenaline in learning: Problem-solving in the marmoset after alpha-noradrenergic receptor blockade. Pharmacology, Biochemistry, and Behavior. 1981;14(6):849–855. doi: 10.1016/0091-3057(81)90373-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiology of Learning and Memory. 2006;86(3):249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Research. 1996;709(2):243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory. 1997;67(2):176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behavioral Neuroscience. 1996;110(5):1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor—cAMP/cAMP/PKA system in influencing memory consolidation. European Journal of Neuroscience. 2002;15(3):553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor—cAMP pathway: Dependence on glucocorticoid receptor activation. Journal of Neuroscience. 2008;28(26):6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. Journal of Comparative Neurology. 1985;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Miasnikov AA, Weinberger NM. Characterisation of multiple physiological fields within the anatomical core of rat auditory cortex. Hearing Research. 2003;181(1–2):116–130. doi: 10.1016/s0378-5955(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex–nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. European Journal of Neuroscience. 2000;12(1):367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106(1):538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic–cholinergic interactions in the primate basal forebrain. Neuroscience. 1999;93(3):817–829. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annual Review of Neuroscience. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Memory codes: New concept for old problem. In: Gold PE, Greenough WT, editors. Memory consolidation: Essays in honor of James L. McGaugh. American Psychological Association; Washington, DC: 2001. pp. 321–342. [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiology of Learning and Memory. 2003;80(3):268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Reviews Neuroscience. 2004;5(4):279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Retuning the brain by learning, literature, and logic: Reply to Suga. Learning & Memory. 2008;15(4):202–207. [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990;1(1):91–132. [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31(5):912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]