Figure 1.
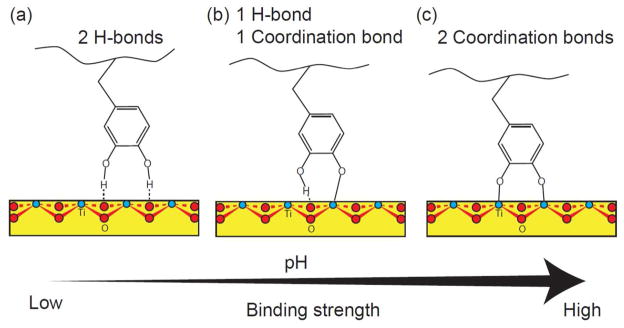
The possible modes of Dopa (catechol) binding to nonhydrated TiO2 surfaces. The catechol group can form molecular adsorption with (a) two hydrogen bonds, (b) monodentate adsorption with one hydrogen bond and one coordination bond, and (c) bidentate adsorption with two coordination bonds. Although which form the Dopa binds to a TiO2 surface depends is pH dependent: at lower pH (pH < 5.5), the molecular adsorption is preferred and at higher pH (pH > 7), the coordination charge transfer is more favorable. As marked, the red atoms are oxygens, the blue ones are titanium.
