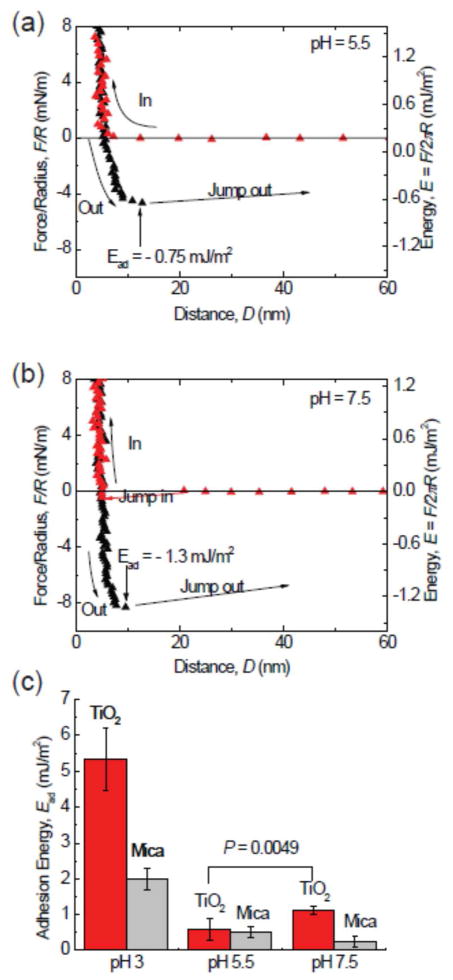
Figure 3.
The pH dependence of adhesion of mfp-3F to TiO2. Reduced adhesion upon increasing the pH to 5.5 (a). A surprising increase in adhesion by almost 40% occurs upon bringing the pH up to 7.5 (b). Although Dopa loss to oxidation is more severe at high pH, the Dopa-TiO2 coordination bond gives higher adhesion forces. (c) A summary of the adhesion energies of mfp-3 on TiO2 in different pH buffers. The adsorption of Dopa on TiO2 surface is highly pH dependent. At low pH, the protonated Dopa predominates, whereas at pH 7.5, there exists a mixture of both half- and fully deprotonated catecholates.