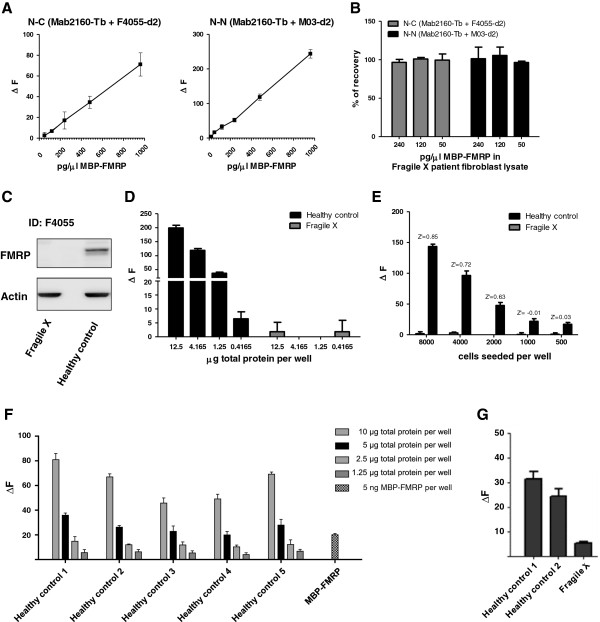
Figure 3.
Assay performance and application for endogenous human FMRP in fibroblasts. (A) Determination of time-resolved Förster’s resonance energy transfer (TR-FRET) assay linearities for purified FMRP when spiked into a fibroblast lysate from a Fragile X syndrome (FragileX) patient without any detectable endogenous FMRP levels. (B) Recovery rate calculations of FMRP protein in FragileX patient lysate based on expected versus quantified FMRP values. (C) Immunoblot for endogenous human FMRP protein in lysates from a healthy control or a FragileX patient. (D) Detection of endogenous human FMRP by the N-N-antibody TR-FRET assay in 5 μl sample volume in low-volume 384-well plate format for serial dilutions of lysates from a healthy control and a FragileX patient. (E) Quantification of endogenous FMRP in healthy control versus FragileX patient fibroblast lines grown and lysed in 384-well plates. (F) Detection of endogenous FMRP in peripheral blood mononuclear cell (PBMC) lysates of healthy human control volunteers by the N-N-antibody combination in the low-volume 384-well plate format. Total protein concentrations per well are indicated. Purified MBP-FMRP protein (5 ng) was used as control. (G) Comparison of FMRP detection signal in PBMC lysates of healthy control patients versus PBMC lysate obtained from a FragileX patient (1.65 μg total protein loaded per well for each sample). Values for A, D, E, F and G presented as percentage signal over assay buffer background. All data and error bars represent averages and standard deviations of triplicates.