Table 2.
Domino Synthesis of Fused Acridines 4 and 5
| entry | product 4 and 5a | substrate (1) | 2(R1) | 3’ | time /min | yield b/% | |
|---|---|---|---|---|---|---|---|
| 1 | 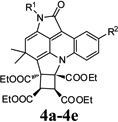 |
4a | Indoline-2,3-dione (1a) | 4-Chlorophenyl (2a) | 3a | 20 | 59 |
| 2 | 4b | Indoline-2,3-dione (1a) | Phenyl (2b) | 3a | 24 | 54 | |
| 3 | 4c | Indoline-2,3-dione (1a) | 4-Tolyl (2c) | 3a | 30 | 61 | |
| 4 | 4d | Indoline-2,3-dione (1a) | 3,4-Dimethoxyphenyl (2d) | 3a | 20 | 50 | |
| 5 | 4e | 5-Fluoroindoline-2,3-dione (1b) | 4-Tolyl (2c) | 3a | 25 | 46 | |
| 6 | 4f | Indoline-2,3-dione (1a) | 4-Chlorophenyl (2a) | 3b | 20 | 53 | |
| 7 | 4g | Indoline-2,3-dione (1a) | Phenyl (2b) | 3b | 22 | 56 | |
| 8 | 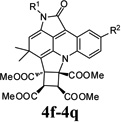 |
4h | Indoline-2,3-dione (1a) | 4-Tolyl (2c) | 3b | 24 | 59 |
| 9 | 4i | Indoline-2,3-dione (1a) | 3,4-Dimethoxyphenyl (2d) | 3b | 26 | 51 | |
| 10 | 4j | Indoline-2,3-dione (1a) | 4-Methoxyphenyl (2e) | 3b | 30 | 48 | |
| 11 | 4k | Indoline-2,3-dione (1a) | Carboxymethyl (2f) | 3b | 28 | 52 | |
| 12 | 41 | 5-Methylindoline-2,3-dione (1c) | 4-Chlorophenyl (2a) | 3b | 26 | 58 | |
| 13 | 4m | 5-Methylindoline-2,3-dione (1c) | Phenyl (2b) | 3b | 25 | 62 | |
| 14 | 4n | 5-Methylindoline-2,3-dione (1c) | 4-Tolyl (2c) | 3b | 26 | 64 | |
| 15 | 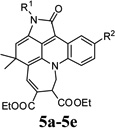 |
4o | 5-Methylindoline-2,3-dione (1c) | 4-Methoxyphenyl (2e) | 3b | 30 | 49 |
| 16 | 4p | 5-Methylindoline-2,3-dione (1c) | 4-Bromophenyl (2g) | 3b | 30 | 52 | |
| 17 | 4q | 5-Chloroindoline-2,3-dione (1d) | 4-Chlorophenyl (2a) | 3b | 28 | 45 | |
| 18 | 5a | Indoline-2,3-dione (1a) | Phenyl (2b) | 3c | 30 | 48 | |
| 19 | 5b | Indoline-2,3-dione (1a) | 4-Tolyl (2c) | 3c | 20 | 51 | |
| 20 | 5c | Indoline-2,3-dione (1a) | 4-Methoxyphenyl (2e) | 3c | 30 | 46 | |
| 21 | 5d | Indoline-2,3-dione (1a) | 3,5-Dichlorophenyl (2g) | 3c | 30 | 43 | |
| 22 | 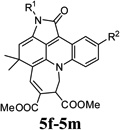 |
5e | Indoline-2,3-dione (1a) | 4-Chlorophenyl (2a) | 3c | 28 | 40 |
| 23 | 5f | Indoline-2,3-dione (1a) | Phenyl (2b) | 3d | 25 | 50 | |
| 24 | 5g | Indoline-2,3-dione (1a) | 4-Tolyl (2c) | 3d | 28 | 52 | |
| 25 | 5h | Indoline-2,3-dione (1a) | 3,4-Dimethoxyphenyl (2d) | 3d | 28 | 45 | |
| 26 | 5i | Indoline-2,3-dione (1a) | 4-Methoxyphenyl (2e) | 3d | 30 | 42 | |
| 27 | 5j | Indoline-2,3-dione (1a) | Carboxymethyl (2f) | 3d | 30 | 46 | |
| 28 | 5k | Indoline-2,3-dione (1a) | 3-Chlorophenyl (2h) | 3d | 28 | 52 | |
| 29 | 51 | 5-Methylindoline-2,3-dione (1c) | 4-Tolyl (2c) | 3d | 28 | 55 | |
| 30 | 5m | 5-Methylindoline-2,3-dione (1c) | 4-Methoxyphenyl (2e) | 3d | 30 | 50 |
Conditions: the synthesis of products 4, (CH3)2CHCOOH (1.5 mL), 90–100 °C, microwave heating; the synthesis of products 5, (CH3)2CHCOOH (1.5 mL), 130–140 °C, microwave heating.
Isolated yield.
