Abstract
Background
The Mirasol pathogen reduction technology (PRT) system uses riboflavin and ultraviolet light and is currently approved and used in Europe for the treatment of platelets and plasma. Mirasol treatment is intended to reduce the infectious pathogen load and to inactivate leukocytes in blood products. Our objective was to evaluate buffy coat platelet concentrates (BCPCs) prepared with platelet additive solution (PAS) and treated with the Mirasol system and to examine the effects on platelet cell quality during storage.
Methods
26 BCPCs were prepared and split, creating 13 paired control and test units. The test units were treated with the Mirasol system and the platelet quality was assessed in all units over 7 days of storage.
Results
All products met the incoming specifications for Mirasol treatment, and the pH of all Mirasol-treated BCPCs in PAS met the requirements of the Council of Europe guidelines throughout storage. Analysis of lactate production and glucose consumption rates, CD62p expression and cytokines indicates enhanced cellular metabolism in treated platelets, but the levels were within previously published ranges.
Conclusion
While Mirasol-treated BCPCs in PAS had increased metabolism and activation compared to controls, the results indicate that these units can be stored for 7 days with acceptable cell quality.
Keywords: Pathogen reduction technology, Mirasol, Buffy coat platelets, PAS-IIIM
Introduction
Blood safety continues to be a topic at the forefront of transfusion medicine. While strict screening criteria and nucleic acid testing (NAT) have significantly reduced the risk of transfusion-related infections, these measures have not completely eliminated the threat of existing or newly emerging pathogens, as evidenced by the recent incident of detection of Trypanosoma rangeli in a blood sample of a blood donor in Valencia, Spain [1]. With the re-emergence of infections such as dengue fever [2] and increasing numbers of outbreaks of pathogens such as chikungunya in areas not previously endemic to such viruses [3], it is likely that reports of this nature will continue to increase as international travel and emigration become commonplace in society. As the mobility of populations increases, so too does the risk of emerging infections in regions that previously were not exposed to these agents.
Over the past several decades, blood donor selection and blood screening practices have become very stringent, culminating in the wide adoption of NAT testing as an additional method for preventing viral transmission by low-viremic or serologically negative blood donations. Yet many of the changes that have occurred in blood banking practices during this time have been reactive, illustrated by the recent discovery of and uncertainty surrounding the xenotropic murine leukemia virus-related virus (XMRV) and its potential threat to the blood supply [4]. The emergence of pathogen reduction technologies (PRTs) represents a new approach to blood safety in that they provide a proactive measure against transfusion-transmitted infections [5]. Pathogen reduction systems have been developed that have proven to be effective against numerous bacteria, viruses, and parasites [6]. Two main systems have emerged to treat platelet concentrates: treatment with psoralen plus ultraviolet A (UVA) light and treatment with riboflavin (vitamin B2) plus UVB light, both targeting the nucleic acids of pathogens. The ability of the Mirasol PRT system to inactivate both pathogens and white blood cells has been previously described [7]. The technology uses a combination of riboflavin and UV light to induce irreversible lesions in the nucleic acids of pathogens and white blood cells (WBCs) to inhibit replication and function.
As one of the principal blood centers in Galicia, Spain, the Centro de Transfusion de Galicia supplies blood to 31 hospitals (17 public, 14 private ones) in the northwest region of Spain which is home to approximately 2.7 million inhabitants. In 2010, the blood center collected 110,000 whole-blood and 8000 apheresis donations. It supplied over 110,000 red blood cell (RBC), 14,700 platelet and 16,600 plasma units to area hospitals [8]. Pathogen inactivation of plasma products prior to transfusion using a methylene blue system (MacoPharma) was implemented in 1998, and platelet concentrates have been treated with the Intercept system (Cerus) since 2008. Given the aforementioned increasing risks to blood donors and recipients previously mentioned, the blood center is investigating available PRTs for platelet products.
The purpose of this study was to evaluate the effects of Mirasol treatment on the cell quality of platelet concentrates produced with an automated device (OrbiSac, Terumo BCT), treated and stored in platelet additive solution (PAS) for 7 days.
Material and Methods
Preparation of Buffy Coat Platelet Concentrates
Whole blood was collected in citrate phosphate dextrose (CPD) (450 ± 45 ml) and rested overnight. The units were then processed on Compomat G4 presses (Fresenius). For platelet concentrate preparation, 5 buffy coats (average 52 ml and 37% hematocrit) were pooled with 250 ml SSP+ (MacoPharma, Malvaux, France) on the OrbiSac system (Terumo BCT, Lakewood, CO, USA). The 26 buffy coat platelet concentrates (BCPCs) were grouped into 13 type-matched pairs, which were pooled and then split, creating 13 untreated control units and 13 (paired) test units.
Treatment of Buffy Coat Platelet Concentrates
Of 500 μmol/l riboflavin solution, 35 ml was added to the test units for treatment with the Mirasol PRT system, and the products were illuminated to 7.7 J/ml plasma. 35 ml of normal saline was added to the untreated control products to mimic the dilution of the test products with riboflavin. All products were stored for 7 days under standard blood banking conditions, with cell quality assays being performed on days 2, 5 and 7 of storage.
Laboratory Methods
Glucose, lactate and lactate dehydrogenase (LDH) were measured using an Olympus AU 400 analyzer. Glucose consumption and lactate production rates over storage were calculated for all products, and are expressed as mmol/1012 cells/h. Platelet concentration and mean platelet volume (MPV) were evaluated using a CBC analyzer (XT-2000i; Sysmex). Measurements for pH were performed at 22 °C (MicropH2001; Crysson). CD62p was measured by flow cytometry (FACSCalibur; Becton Dickinson). Platelet swirling was assessed visually and given a numeric score (range 0–3) based on previously described methods [9].
Supernatants from the platelet products were used for cytokine analyses. Supernatants were prepared via centrifugation and stored at −80 °C until assayed. Enzyme-linked immunosorbent assay (ELISA) kits for RANTES (regulated upon activation normal ? cell expressed and presumably secreted), sCD62p, and transforming growth factor β-1 (TGFβ-1) (R&D Systems, Minneapolis, MN, USA) were used, according to the manufacturer's instructions. Samples were analyzed in duplicate.
Statistical Analysis
Statistical analyses of the data were performed using GraphPad software (version 5.04; La Jolla, CA, USA). Statistical significance of the cell quality data was determined using a two-way repeated measures (RM) analysis of variance (ANOVA) to evaluate differences between untreated control and Mirasol-treated units over the entire storage period. In addition, post-tests using the Bonferroni method were performed for each data set. Glucose consumption and lactate production rates were analyzed using the Deming linear regression model. For all methods of analysis, p values < 0.05 were considered significant. Data are expressed as mean ± standard deviation.
Results
All 26 BCPCs met the incoming product specifications for Mirasol treatment: The average product volume was 319 ± 8 ml, with a mean plasma carryover of 40.8 ± 2.6% and a platelet concentration of (1,144 ± 117) × 106/ml.
The cell quality measurements for the control and test units on days 2, 5 and 7 are summarized in table 1.
Table 1.
Summary of in vitro cell quality parameters
| Parameter | Storage day | ||
|---|---|---|---|
| 2 | 5 | 7 | |
| pH at 22 °C | |||
| C | 7.31 ± 0.04 | 7.36 ± 0.09 | 7.35 ± 0.08 |
| M | 7.23 ± 0.02 | 7.10 ± 0.13* | 7.02 ± 0.08* |
| Platelet concentration | |||
| C | 1076 ± 78 | 1073 ± 68 | 1068 ± 78 |
| M | 1079 ± 69 | 1050 ± 67 | 1062 ± 80 |
| Swirl | |||
| C | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| M | 3.0 ± 0.0 | 3.0 ± 0.2 | 2.4 ± 0.5* |
| Lactate, mmol/1012 cells | |||
| C | 4.6 ± 0.8 | 8.1 ± 0.9 | 10.5 ± 1.8 |
| M | 5.4 ± 1.0 | 11.3 ± 1.5* | 15.8 ± 1.4* |
| Glucose, mmol/1012 cells | |||
| C | 5.8 ± 1.1 | 4.1 ± 1.3 | 3.0 ± 1.3 |
| M | 5.6 ± 1.1 | 3.1 ± 1.2* | 0.8 ± 0.7* |
| LDH, IU/1 | |||
| C | 72.4 ± 20.4 | 99.2 ± 18.2 | 94.0 ± 19.0 |
| M | 72.7 ± 15.2 | 94.5 ± 11.9 | 127.3 ± 20.9* |
| MPV, fl | |||
| C | 8.8 ± 0.2 | 8.9 ± 0.3 | 9.1 ± 0.6 |
| M | 8.8 ± 0.3 | 9.4 ± 0.3* | 10.1 ± 0.4* |
| CD62p, % expression | |||
| C | 17.6 ± 6.3 | 18.8 ± 7.0 | 18.1 ± 3.3 |
| M | 50.9 ± 3.4* | 55.7 ± 3.6* | 57.2 ± 8.4* |
| Annexin V, % binding | |||
| C | 3.2 ± 1.7 | 3.5 ± 1.6 | 4.0 ± 1.2 |
| M | 4.1 ± 2.8 | 5.2 ± 1.1 | 12.2 ± 5.7* |
| sCD62P, ng/ml | |||
| C | 61.2 ± 40.2 | 78.8 ± 39.2 | 110.6 ± 49.3 |
| M | 47.4 ± 19.2 | 101.3 ± 38.6 | 140.6 ± 40.0* |
| RANTES, ng/ml | |||
| C | 419.1 ± 98.9 | 481.5 ± 206.4 | 449.4 ± 166.6 |
| M | 398.4 ± 270.5 | 396.4 ± 167.4 | 486.1 ± 162.5 |
| TGFβ-1, ng/ml | |||
| C | 6.6 ± 6.9 | 14.6 ± 18.0 | 16.0 ± 16.8 |
| M | 38.7 ± 19.7* | 61.9 ± 14.3* | 73.0 ± 18.1* |
denotes statistical significance between groups.
C = Control
M = Mirasol-treated.
The pH of all units decreased over the 7-day storage period, but remained well within the Council of Europe guidelines for product release (> 6.4) [10]. In addition, all platelet concentrates met this criterion on day 9 of storage (data not shown).
Elevated metabolism was observed in Mirasol-treated BCPCs, as demonstrated by increased glucose consumption and lactate production rates compared to controls over the course of storage (fig. 1 and 2), which were, however, well within the limits previously established for acceptable in vivo recovery and survival [11]. LDH measurements yielded comparable results for control and test units through day 5, with a slight increase in Mirasol-treated units observed on day 7.
Fig. 1.
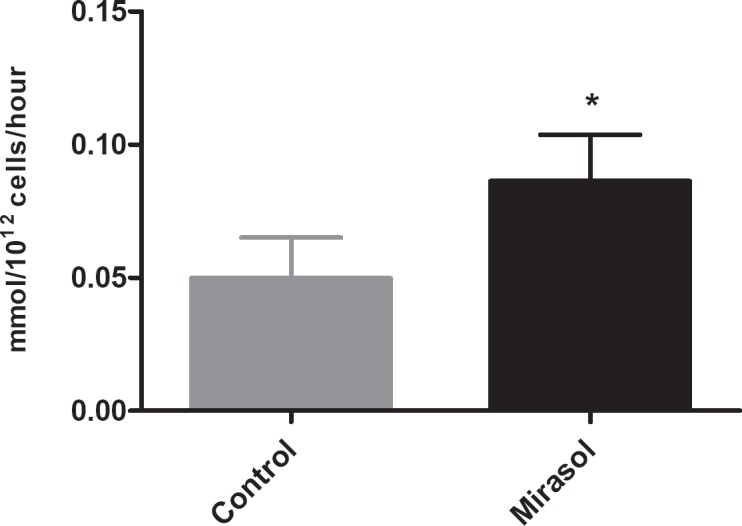
Lactate production rate over 7 days of storage; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets; (*) indicates statistical significance between groups.
Fig. 2.
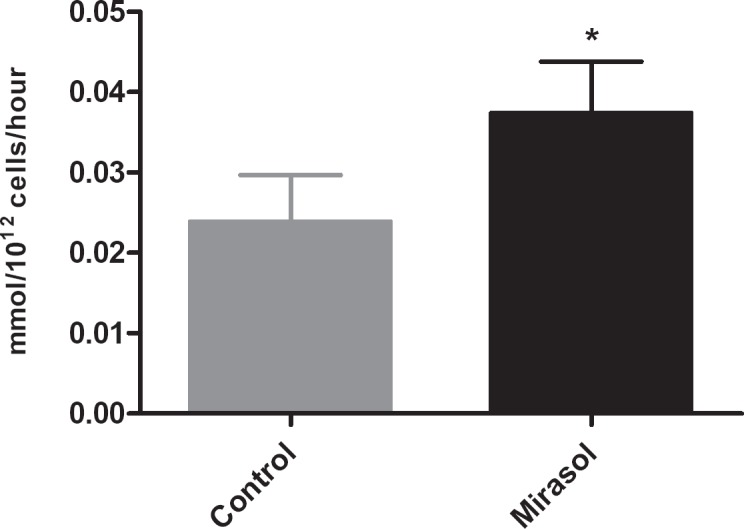
Glucose consumption rate over 7 days of storage; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets; (*) indicates statistical significance between groups.
Swirl scores were slightly decreased in Mirasol-treated units on days 5 and 7 of storage, but all units maintained positive swirl up to day 7. This corresponded to a slight increase in MPV of the Mirasol units compared to the control units.
The platelet concentration remained stable in all units until the end of storage, and was comparable in control and Mirasol-treated BCPCs (fig. 3).
Fig. 3.
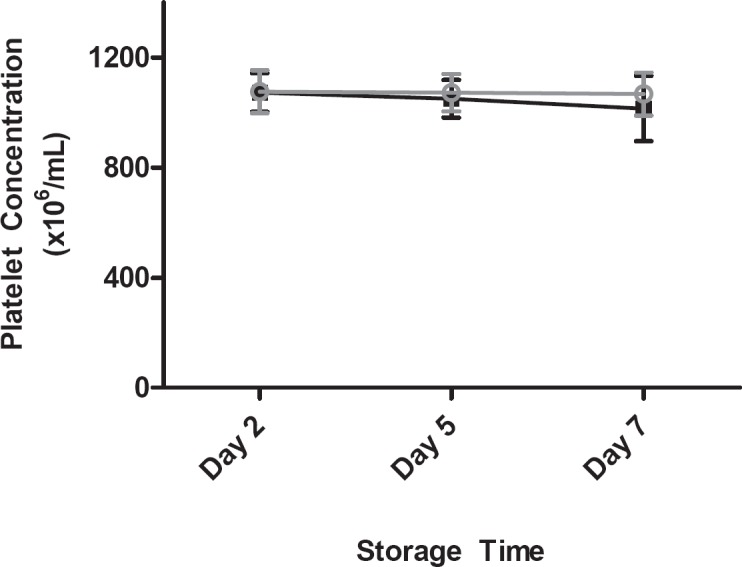
Platelet count as a function of storage time; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets.
As has been previously reported [12], CD62p expression was elevated in the Mirasol-treated units compared to untreated control units, although the total increase over the storage period was small (fig. 4). Correspondingly, levels of soluble CD62p were also elevated in Mirasol units.
Fig. 4.
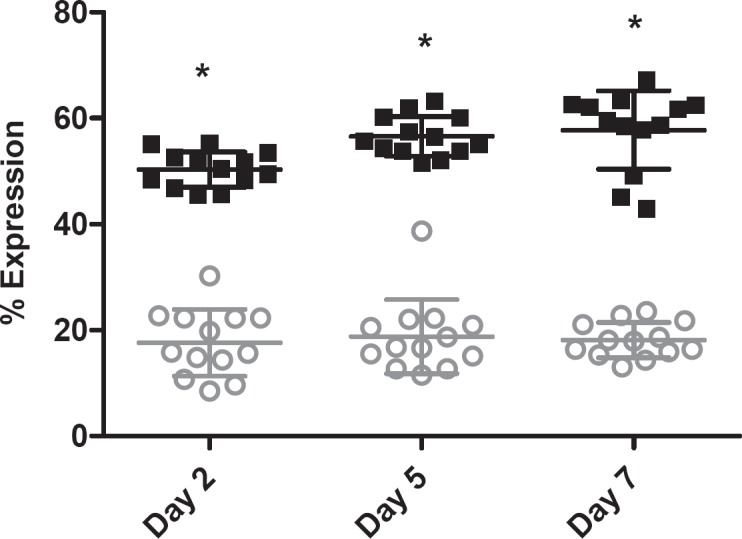
CD62p expression as a function of storage time; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets; (*) indicates statistical significance between groups.
Annexin V binding was similar in Mirasol and control BCPCs through 5 days of storage, reaching statistical significance only on day 7 when it was greater in the Mirasol units.
Cytokine analyses revealed that, compared to controls, Mirasol-treated platelet concentrates had increased levels of TGFβ-1 at all time points and increased levels of soluble CD62p on days 5 and 7 of storage (fig. 5). However, while the concentration of RANTES increased slightly in all units throughout storage, the values for control and Mirasol BCPCs were statistically indiscernible (fig. 6).
Fig. 5.
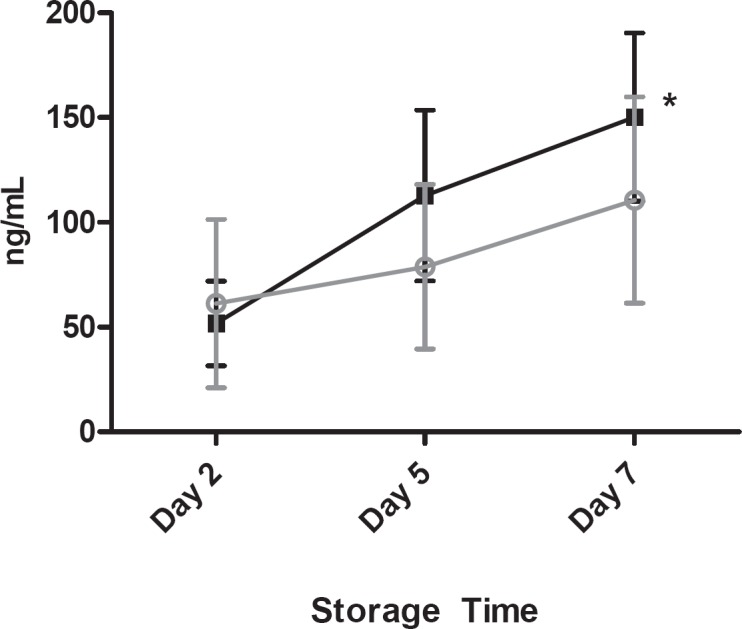
Soluble CD62p as a function of storage time; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets; (*) indicates statistical significance between groups.
Fig. 6.
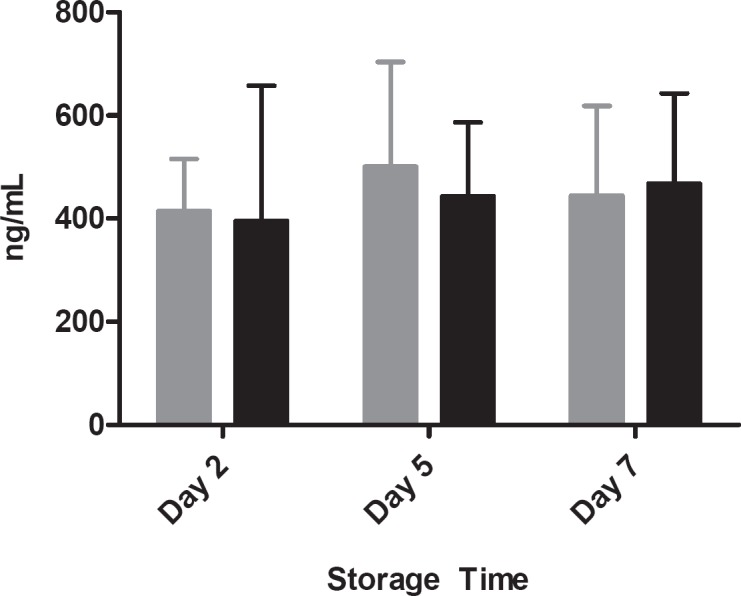
RANTES concentration as a function of storage time; error bars indicate 1 standard deviation. (▪) Control platelets, (▪) Mirasol-treated platelets.
Discussion
Overall, the platelet concentrates in PAS treated with the Mirasol system evaluated in this study demonstrated minimal platelet loss due to treatment and acceptable cell quality through 7 days of storage. In addition, only a slight decrease in swirl was observed on the last day of storage, indicating that platelet morphology was largely preserved in all units. Comparable LDH concentrations between the 2 groups during storage would also suggest that the membranes of Mirasol platelets remain intact following treatment, as leakage of LDH only appeared to increase at the very end of storage.
Lactate production and glucose consumption were elevated in Mirasol-treated platelets (which also accounts for the slightly lower pH observed in these units), but remained within previously published ranges that yield acceptable survival and recovery [11,13]. Mirasol-treated platelets appeared to be more activated than untreated controls, as evidenced by elevated levels of CD62p expression on the cell surface at all storage time points. However, soluble CD62p was statistically greater in Mirasol-treated units only at the end of storage. Annexin V binding was statistically lower in control units only on day 7. It is noteworthy that the overall annexin V levels measured for all units in this study remained on average below 12%. These results are similar to those observed in previous studies. The day 7 P-selectin values reported by both Ostrowski et al. [14] and Reikvam et al. [15] are comparable to those observed in our study, as are the day 7 annexin V data from the Ostrowski group. Both groups also observed increased metabolism in Mirasol-treated platelets. In this current study, the final platelet concentrates were produced by an automated device, but this method did not appear to have any additional effect on the in vitro quality of platelets when compared to those previous results.
Interestingly, however, when Ostrowski et al. [16] analyzed Mirasol-treated platelet concentrates using thromboelastography (TEG), they reported that the capacity of clot formation was retained and differed very little from that of untreated platelet concentrates, suggesting that, despite the differences observed in in vitro cell activation and metabolism, the hemostatic function of Mirasol-treated platelets is maintained. Michelson et al. [17] have found that even highly activated platelets, demonstrated by P-selectin expression and glycoprotein (GP) Ilb/IIIa, still circulate and function in 3 different animal coagulation models.
Secretion of the cytokine TFGβ-1 was visibly increased in the Mirasol-treated units, but interestingly there was no statistically significant difference in levels of RANTES at any time point between the control and Mirasol-treated platelet concentrates. While RANTES is associated with allergic reactions in patients following platelet transfusions [18], the data here suggest that it is not likely that Mirasol treatment would cause an increase in such reactions compared to standard platelet products. This assessment is also supported by recently published clinical data for Mirasol-treated platelet concentrates [19], where no adverse events attributed to the use of the Mirasol system occurred. Furthermore, post-market surveillance and complaint handling systems have to date received no reports of any additional adverse events or reportable events that a physician has attributed to the Mirasol system. To our knowledge, this is the first study published in which cytokine levels were extensively monitored during storage of platelet concentrates produced by an automated device (OrbiSac) and treated with the Mirasol system.
Although the adoption of PRTs has been recommended by international experts since the initial discussions during the Canadian Consensus Conference [20], the actual adoption of such technologies has been very slow. One explanation for this might be the difficulty of estimating overall public health cost savings achieved by reducing a theoretical risk of infection and allo-immunization to individuals. On the other hand, hospitals and health care providers use the direct cost associated with PRTs to support their decision making process. In conclusion, understanding the overall public health gain is one of the major challenges for broad PRT adoption. However, recent analyses have demonstrated that PRT-treated blood products may actually reduce the overall health care costs [21] and the duration of hospital stay associated with post-transfusion care for some patients. Due to the ability of the Mirasol PRT treatment to inactivate all remaining leukocytes in a product that mediate graft-versus-host disease (GvHD) [22], it can be used as an alternative to γ-irradiation. γ-Irradiation is both costly and cumbersome for hospitals to maintain and operate [23]. In addition, the Mirasol PRT system, but not γ-irradiation which is considered to be the gold standard to inactivate residual leukocytes in blood products, has been shown to prevent accumulation and secretion of most WBC-associated cytokines [24], with the potential to prevent leukocyte-mediated immunologic reactions in recipients [25]. Benefits of the Mirasol process include the use of a natural nontoxic compound (riboflavin) as the activating agent and a short (4–6 min) illumination process after which the products can be immediately released for transfusion. The Mirasol system is easy to use and does not require special training to operate the equipment. This technology can eliminate most of the residual risk of bacteria and also reduces the risk associated with a long list of transfusion-transmitted pathogens for which the blood supply is not screened.
Conclusions
Treatment of platelet concentrates with the Mirasol PRT system increases cellular metabolism (as evidenced by increased lactate production and glucose consumption rates) and the expression of platelet activation markers such as P-selectin. However, these BCPCs demonstrate acceptable cell quality through 7 days of storage, falling within previously reported ranges for acceptable in vivo survival and recovery. Clinical studies to evaluate the clinical effectiveness of Mirasol-treated platelets in PAS are ongoing.
Disclosure Statement
AC has no conflict of interests. MC and LR are employees of Terumo BCT.
References
- 1.Parada C, Villalba J, Alvarez M, Puig N, Planelles D, Ramada C, Montoro J, Roig R. Trypanosoma rangeli in a blood donor at the Valencian Blood Transfusion Centre. Vox Sang. 2010;99:193–194. doi: 10.1111/j.1423-0410.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Brouard C, Bernillon P, Quatresous I, Pillonel J, Assal A, de VH, Desenclos JC. Estimated risk of chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. 2008;48:1333–1341. doi: 10.1111/j.1537-2995.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 4.Dodd RY. XMRV, Q fever and other emerging infections: impact on management of blood safety. ISBT Sci Ser. 2011;6:119–123. [Google Scholar]
- 5.AuBuchon JP, Prowse CV, editors. Pathogen Inactivation: The Penultimate Paradigm Shift. Bethesda: AABB Press; 2010. [Google Scholar]
- 6.Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Marschner S, Goodrich R. Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus Med Hemother. 2011;38:8–18. doi: 10.1159/000324160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centro de Transfusion de Galicia : Annual Report 2010. http://ctg.sergas.es/
- 9.Ross D, Holme S, Hartman P, Reece K, Oubilla L, Heaton A. A quick visual method for quality control of platelet concentrates. Transfusion. 1986;26:550. [Google Scholar]
- 10.Guide to the Preparation, Use and Quality Assurance of Blood Components. Strasbourg: Council of Europe; 2011. [Google Scholar]
- 11.Goodrich RP, Li J, Pieters H, Crookes R, Roodt J, Heyns A. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–285. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 12.Picker SM, Oustianskaia L, Schneider V, Gathof BS. Functional characteristics of apheresis-derived platelets treated with ultraviolet light combined with either amotosalen-HCl (S-59) or riboflavin (vitamin B2) for pathogen-reduction. Vox Sang. 2009;97:26–33. doi: 10.1111/j.1423-0410.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 13.AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, Edrich R, Goodrich RP. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45:1335–1341. doi: 10.1111/j.1537-2995.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski SR, Bochsen L, Salado-Jimena JA, Ullum H, Reynaerts I, Goodrich RP, Johansson PI. In vitro cell quality of buffy coat platelets in additive solution treated with pathogen reduction technology. Transfusion. 2010;50:2210–2219. doi: 10.1111/j.1537-2995.2010.02681.x. [DOI] [PubMed] [Google Scholar]
- 15.Reikvam H, Marschner S, Apelseth TO, Goodrich R, Hervig T. The Mirasol Pathogen Reduction Technology system and quality of platelets stored in platelet additive solution. Blood Transfus. 2010;8:186–192. doi: 10.2450/2010.0141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Bochsen L, Windelov NA, Salado-Jimena JA, Reynaerts I, Goodrich RP, Johansson PI. Hemostatic function of buffy coat platelets in additive solution treated with pathogen reduction technology. Transfusion. 2011;51:344–356. doi: 10.1111/j.1537-2995.2010.02821.x. [DOI] [PubMed] [Google Scholar]
- 17.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluter H, Bubel S, Kirchner H, Wilhelm D. Febrile and allergic transfusion reactions after the transfusion of white cell-poor platelet preparations. Transfusion. 1999;39:1179–1184. doi: 10.1046/j.1537-2995.1999.39111179.x. [DOI] [PubMed] [Google Scholar]
- 19.Mirasol Clinical Evaluation Study Group A Randomized controlled clinical trial evaluating the performance and safety of platelets treated with Mirasol pathogen reduction technology. Transfusion. 2010;50:2362–2375. doi: 10.1111/j.1537-2995.2010.02694.x. [DOI] [PubMed] [Google Scholar]
- 20.Webert KE, Cserti CM, Hannon J, Lin Y, Pavenski K, Pendergrast JM, Blajchman MA. Proceedings of a consensus conference: pathogen inactivationmaking decisions about new technologies. Transfus Med Rev. 2008;22:1–34. doi: 10.1016/j.tmrv.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Custer B, Agapova M, Martinez RH. The cost-effectiveness of pathogen reduction technology as assessed using a multiple risk reduction model. Transfusion. 2010;50:2461–2473. doi: 10.1111/j.1537-2995.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 22.Marschner S, Fast LD, Baldwin WM III, Slichter SJ, Goodrich RP. White blood cell inactivation after treatment with riboflavin and ultraviolet light. Transfusion. 2010;50:2489–2498. doi: 10.1111/j.1537-2995.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 23.Mintz PD. Cesium cessation? An advantage of pathogen reduction treatments. Transfusion. 2011;51:1369–1376. doi: 10.1111/j.1537-2995.2011.03201.x. [DOI] [PubMed] [Google Scholar]
- 24.Fast LD, DiLeone G, Marschner S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion. 2011;51:1397–1404. doi: 10.1111/j.1537-2995.2010.02984.x. [DOI] [PubMed] [Google Scholar]
- 25.Asano H, Lee CY, Fox-Talbot K, Koh CM, Erdinc MM, Marschner S, Keil S, Goodrich RP, Baldwin WM. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation. 2007;84:1174–1182. doi: 10.1097/01.tp.0000287318.94088.d7. [DOI] [PubMed] [Google Scholar]


