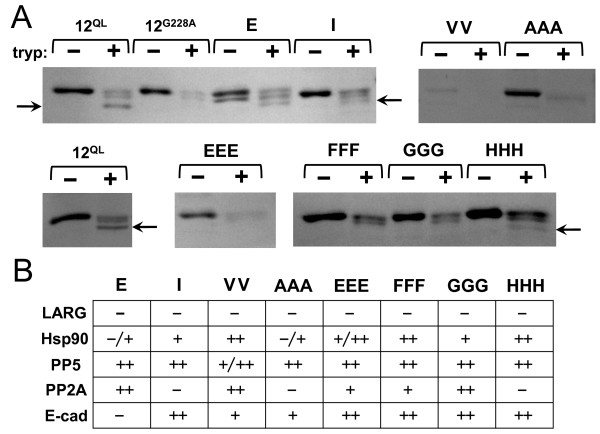
Figure 5.
Conformational efficacy of N-terminal and C-terminal Gα12 mutants uncoupled from RhoGEFs. (A) Trypsin protection of selected Gα12 mutants. HEK293 cell lysates expressing the indicated variants of myc-Gα12QL, or unmodified myc-Gα12QL (12QL), or the G228A variant of myc-Gα12 (12G228A) were subjected to trypsin protection assays as described in Methods. Samples were incubated 20 min at 30°C in the presence (+) or absence (−) of TPCK-treated trypsin, and were analyzed by SDS-PAGE and immunoblotting using J169 antibody (1:700 dilution). Small horizontal arrows indicate position of the trypsin-protected fragment in selected lanes. Data presented are representative of two or more independent experiments per sample. (B) Specificity of uncoupling in selected Gα12 variants. For each cassette mutant of myc-Gα12QL (indicated at top), interaction with each Gα12 target (indicated at left) was quantified as a pulldown:load ratio as described in Methods, and was calculated as a percent of the identical ratio determined for myc-Gα12QL within the same experiment. Values are indicated as follows: (++) = >60%, (+) = 20 to 60%, (−) = 0 to 20%. Interacting proteins are GST fusions of the following: RH domain of LARG (LARG), C-terminal 107 amino acids of heat shock protein-90 alpha (Hsp90), protein phosphatase-5 (PP5), scaffolding Aα subunit of protein phosphatase-2A (PP2A), C-terminal 98 amino acids of E-cadherin (E-cad). Values presented indicate the mean of two or more trials per interaction sample.