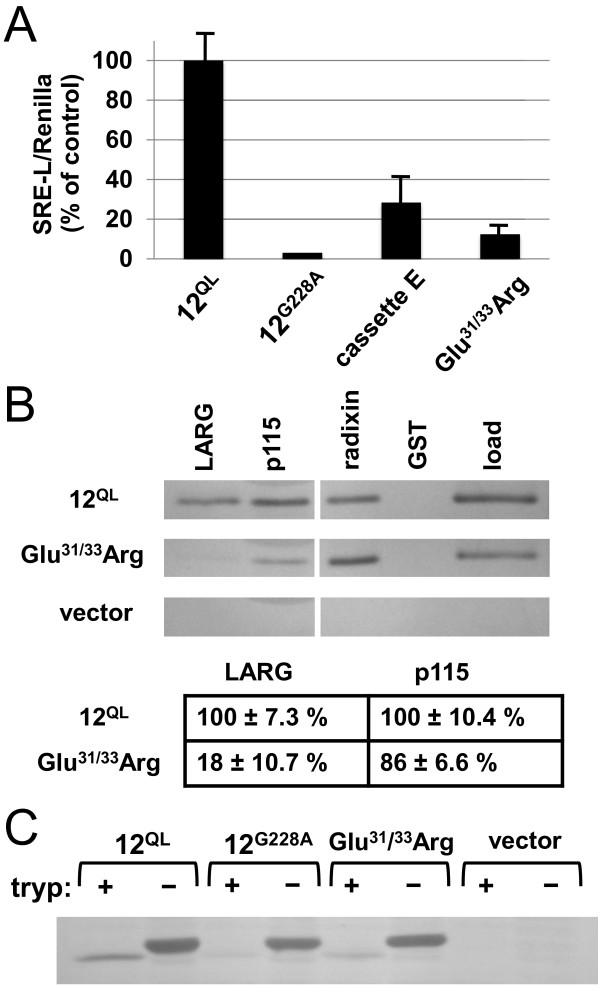
Figure 7.
Selective RhoGEF uncoupling by N-terminal charge substitutions in Gα12. (A) Luciferase reporter gene assays. Cassette mutant E (see Figure 2) and the double charge substitution mutant Glu31/33Arg were compared to myc-Gα12QL (12QL) in SRE-luciferase assays under the cell transfection conditions described in Figure 4. A constitutively GDP-bound variant of wildtype myc-Gα12 (12G228A) was assayed in parallel as a negative control. Results shown are the mean of three independent experiments, and error bars indicate range. (B) Protein-protein interaction assays. Detergent-soluble extracts from transfected HEK293 cells transfected with myc-Gα12QL, the Glu31/33Arg mutant, or empty pcDNA3.1 plasmid (vector) were subjected to co-precipitation assays as described in Methods, using GST-fusions of either LARG-RH (LARG), p115RhoGEF-RH (p115), the N-terminal domain of the Gα12 target radixin [46], or no adduct (GST). Prior to the precipitation step, 5% of each lysate was set aside as starting material (load). Table values show the pulldown:load ratio for Glu31/33Arg as a percent of the positive control value (12QL), with mean +/- range presented for three independent experiments. (C) Trypsin protection of the Glu31/33Arg mutant, in comparison to constitutively GTP- and GDP-bound Gα12. Assays were performed as described in Methods. Results shown are representative of two independent experiments.