Figure 2. Genetic and Taqman qPCR assays used to identify E. coli mutants deficient in retrohoming.
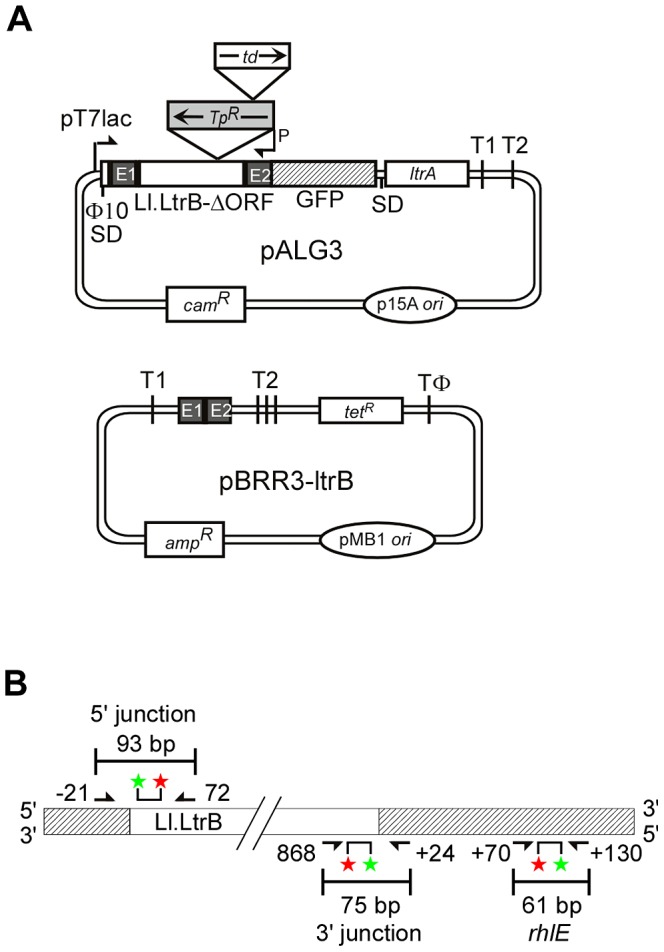
(A) Genetic assay. The CamR intron-donor plasmid pALG3 uses a T7lac promoter and phage Φ10 Shine-Dalgarno (SD) sequence to express an ltrB/GFP fusion cassette. This cassette consists of a 0.9-kb Ll.LtrB-ΔORF intron and flanking 5′- and 3′-exons (E1 and E2, respectively) [19], with the intron carrying a trimethoprim-resistance retrotransposition-activated genetic marker (TpR-RAM), and E2 linked in-frame to an ORF encoding GFP. The LtrA ORF preceded by its own Shine-Dalgarno sequence is co-transcribed from a position downstream of the GFP ORF. The AmpR recipient plasmid contains a 45-bp Ll.LtrB target site (ligated E1–E2 sequence) upstream of a promoterless tetR gene. T1 and T2 are E. coli rrnB transcription terminators, and TΦ is a phage T7 transcription terminator. (B) Taqman qPCR assay. The assay quantifies 5′- and 3′-intron integration junctions resulting from retrohoming of a retargeted Ll.LtrB-ΔORF intron into a site in the rhlE gene in the E. coli chromosome. Retrohoming events are quantified by Taqman qPCR, which utilizes the 5′→3′ exonuclease activity of Taq DNA polymerase to cleave a fluorescently labeled DNA probe that base pairs to an internal region of a PCR amplicon. Digestion of the probe by Taq DNA polymerase releases the FAM label (red star) free of the MGB quencher (green star), resulting in a quantifiable fluorescence signal for each amplification event. The numbers of 5′- and 3′-intron integration junctions relative to the number of rhlE targets were determined by quantifying the fluorescence signals in three separate PCRs relative to standard curves generated from serial dilutions of a reference plasmid (Materials and Methods). Primers for these PCRs are depicted by arrows with numbers indicating the positions of the 5′ nucleotide of the upstream primer and 3′ nucleotide of the downstream primer relative to the intron-integration junction.
