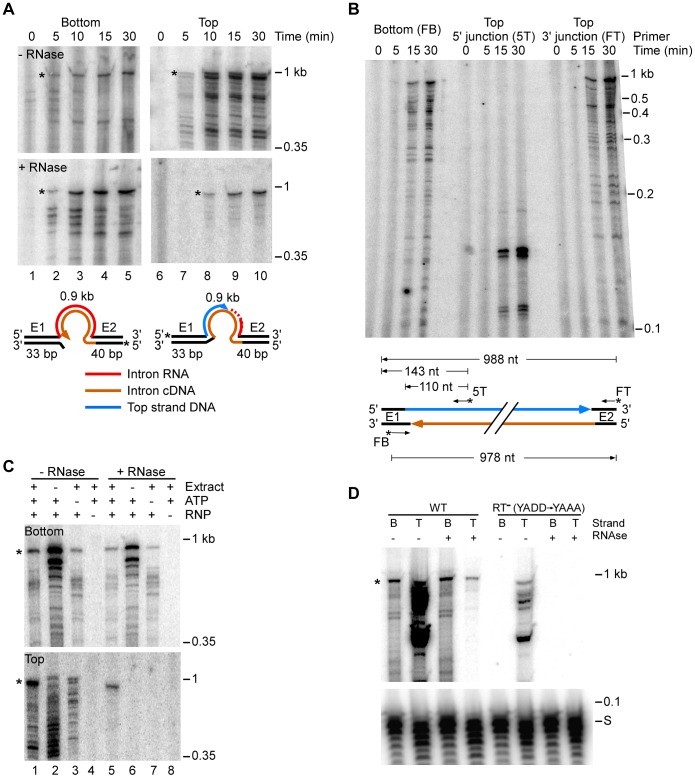
Figure 3. E. coli extract assay for bottom-strand (cDNA) and top-strand DNA synthesis.
(A) Time courses. Group II intron RNPs and labeled DNA substrates (73 bp) containing the Ll.LtrB-insertion site (ligated E1–E2 sequence) were incubated with E. coli HMS174(DE3) extract in the presence of 1 mM dNTPs, 1.5 mM ATP, and an ATP-regenerating system (phosphoenolpyruvate+pyruvate kinase) at 37°C. The DNA substrates were labeled at the 5′ end of either the top (T) or bottom (B) strands to separately assay top- and bottom-strand DNA synthesis. After terminating portions of the reaction at the indicated times, samples were split into halves, which were incubated without or with RNases A+H, and the products were analyzed in a denaturing 6% polyacrylamide gel, which was dried and scanned with a PhosphorImager. RNase-sensitive top-strand products contain the reverse-spliced intron RNA. Schematics below the gels depict bottom- and top-strand synthesis on the DNA substrates (intron and exons not drawn to scale; star indicates 5′ 32P-label). (B) Primer extension analysis. DNA products synthesized in a time course were digested with RNase A+H, purified in a 1% agarose gel (0.85–1.2 kb gel slice), and analyzed by primer extension using 5′ -labeled primers to detect bottom-strand cDNAs (primer FB); the top-strand 5′-intron-integration junction (primer 5T); and top-strand DNAs (primer FT). Major products are diagrammed below the gel. (C) Requirements for bottom- and top-strand DNA synthesis. Reactions with the indicated components were incubated at 37°C for 30 min and then processed and analyzed in a denaturing 6% polyacrylamide gel, as described above. (D) Bottom- and top-strand products obtained with RNPs containing wild-type LtrA protein or an RT-deficient mutant LtrA (RT−; YADD motif changed to YAAA). For simplicity, the bottom part of the gel with the labeled DNA substrate (S) is shown only for panel D. Asterisks indicate gels bands of the size expected for full-length bottom- and top-strand products.