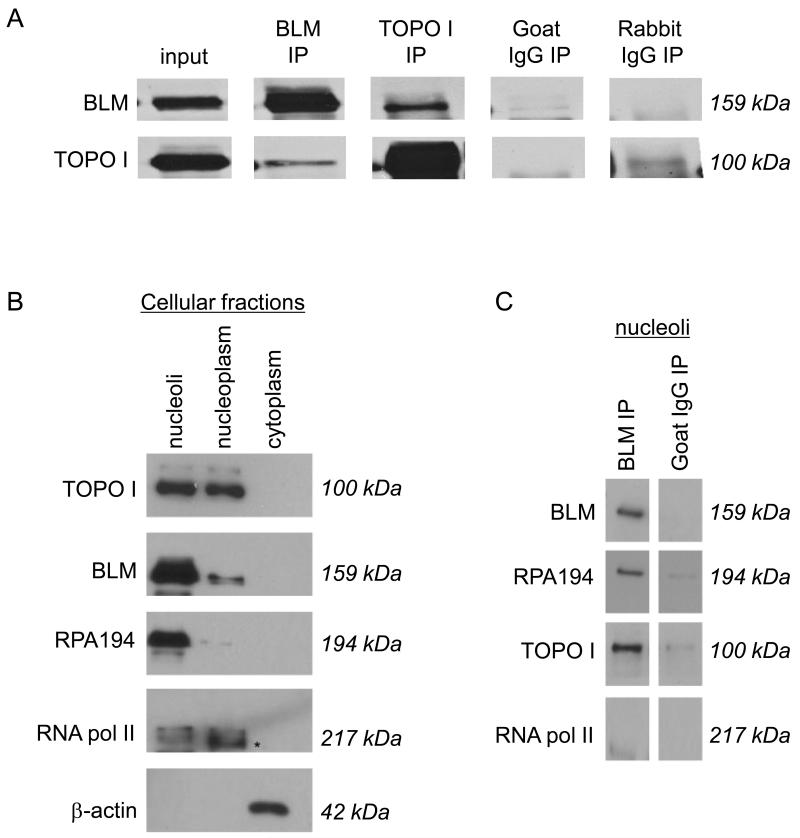
Figure 1. BLM and DNA topoisomerase I associate in mammalian cells.
(A) Co-immunoprecipitations used MCF7 and 293T nuclear extracts, and either anti-BLM or anti-DNA topoisomerase I antibodies (referred as α BLM or α DNA topoisomerase I respectively). Immunoprecipitated proteins were separated using 8% SDS-PAGE and detected by western blotting using α BLM or α DNA topoisomerase I. Goat IgG was chosen as an isotype-matched negative control for α BLM; rabbit IgG was an isotype-matched negative control for α DNA topoisomerase I. (B) 293T nuclei were sub-fractionated into nucleoli and nucleoplasm. The purity of the sub-cellular fractions is shown using the RNA polymerase I-specific subunit RPA194 as a nucleolar marker; RNA polymerase II as a nucleoplasmic marker (indicated with an *); and α -actin as a cytoplasmic marker. Proteins were separated using 8% SDS-PAGE and detected by western blotting using the respective antibodies as indicated. (C) Nucleolar fractions were used in protein co-immunoprecipitation with α BLM. Immunoprecipitated proteins were separated using 8% SDS-PAGE and detected by western blotting using α BLM, α DNA topoisomerase I, α RPA194 and α RNA polymerase II. Goat IgG was used as an isotype-matched negative control for α BLM.