Abstract
Objectives
The purpose of this study was to explore the relationship between left ventricular (LV) dysfunction and arterial wall stiffening.
Methods
A total of 218 patients over the age of 45 diagnosed with hypertension in Jinan City and hospitalised between 2010 and 2011 were included in this study. LV function was evaluated using echocardiography (ECHO). Blood pressure was monitored with an automated tonometric device, and the parameters of arterial wall stiffness were measured. In addition, the metabolic parameters of blood samples, such as glucose and lipids, were also determined using the Cobas E601 analyser.
Results
Stiffness parameter beta positively correlated with LV diastolic function (E/Em ratio) (r = 0.255, p < 0.001). LV end-diastolic diameter not only related to the E/Em ratio (r = 0.196, p = 0.009) but also with beta (r = 0.220, p = 0.002). The stiffness parameter beta was an early indicator of E/Em ratio as determined by multiple regression analysis (R2 = 0.381, p < 0.01). Age, blood pressure and fasting blood glucose contributed to stiffness parameter beta (p < 0.05), as well as the E/Em ratio (p < 0.01).
Conclusions
Our findings suggested that LV dysfunction may have a direct relationship to arterial stiffening, independently of having similar risk factors. In addition, arterial stiffness can be an independent predictor of LV diastolic function, suggesting that the severity of arterial stiffness directly correlates with the severity of LV dysfunction.
Keywords: Arterial stiffness; Left diastolic function; Heart failure, Echocardiography
Introduction
Heart failure is a major cause of cardiovascular morbidity and mortality. Heart failure with a preserved ejection fraction (HFPEF) is becoming more prevalent than heart failure accompanied by systolic impairment [1]. The pathogenesis of HFPEF remains unclear. Clinically, LV diastolic dysfunction often occurs with advanced age, hypertension, and diabetes, which are all associated with an increased arterial stiffness. This could prompt a possibly homologous disease of arterial-myocardial stiffening. However, few studies have explored the common risk factors of concurrent arterial stiffening and LV diastolic function. Some evidence suggested that HFPEF may be the result of interplay between ventricular and vascular stiffening [2, 3]. This coupling of ventricular and vascular stiffening may lead to load-dependent impairment of systolic function as well as diastolic ventricular function [3–5]. The process of vascular stiffening and ventricular dysfunction begin a significant period of time before symptoms occur. Accordingly, it is believed that early detection of changes in arterial and left ventricular properties may be useful in preventing the development of severe cardiovascular complications [6].
The aim of this study was to explore the relationship between the left ventricular (LV) dysfunction and arterial wall stiffening. The goals were to determine the relationship of arterial stiffness and LV diastolic function to demographic variables, LV structural and systolic functional parameters, levels of blood pressure, blood lipids, blood glucose and other measures of metabolism. Furthermore, this study also aimed to assess common risk factors of ventriculo-vascular stiffening and the pathogenesis of HFPEF.
Methods
Study population
A health survey was performed in Jinan City, China, whose inhabitants were homogeneous Chinese. Subjects gave signed consent that permitted the use of collected data for research without disclosure of personal identity. The study protocol was approved by the Ethics Committee of Shandong University Qilu Hospital, China. Systolic arterial blood pressure >140 mmHg or diastolic arterial blood pressure >90 mmHg were defined as hypertension.
Of the target population of 4985, 1080 patients suffered from hypertension, 198 of whom were not receiving any antihypertensive medication. All the participants were screened for arterial stiffness, echocardiographic measurements and serum biochemistry parameters. We excluded patients with abnormal sinus rhythm, regional wall motion abnormalities due to coronary arterial disease, mitral or aortic stenosis, more than mild valvular regurgitation, or with a decreased ejection fraction (EF < 50 %) regardless of cause.
Demographic data and medical history for each subject were obtained during a face-to-face interview using a structured questionnaire. Anthropometric parameters including weight, height, waist-and-hip circumference were measured. Characteristics of the study population are listed in Table 1.
Table 1.
Characteristics of all eligible subjects (mean ± SD)
| Characteristic | Patients (n = 198) |
|---|---|
| Age (years) | 54.3 ± 9.1 |
| BMI (kg/m2) | 25.3 ± 4.4 |
| Sex (women) | 53 (0.366) |
| Waist-to-hip ratio | 0.88 ± 0.04 |
| Systolic blood pressure (mmHg) | 145 ± 18 |
| Diastolic blood pressure (mmHg) | 83 ± 13 |
| Pulse pressure (mmHg) | 65 ± 11 |
| Resting heart rate (/min) | 77 ± 12 |
| High-density lipoprotein cholesterol (mmol/l) | 1.1 ± 0.4 |
| Low-density lipoprotein cholesterol (mmol/l) | 3.5 ± 0.8 |
| Triglycerides (mmol/l) | 1.7 ± 0.6 |
| Cholesterol (mmol/l) | 5.0 ± 0.8 |
| Fasting blood glucose (mmol/l) | 5.7 ± 1.1 |
| Creatinine (μmol/l) | 80.8 ± 16.1 |
| Uric acid (mmol/l) | 339.3 ± 71.2 |
| Stiffness parameter beta | 13.8 ± 6.3 |
| LV ejection fraction (%) | 65.7 ± 9.3 |
| E/Em ratio | 14.5 ± 3.3 |
| end-diastolic thickness of LV septum (mm) | 1.3 ± 0.3 |
| LV end-diastolic diameter (cm) | 4.8 ± 0.8 |
| LV mass index (g/m2) | 133 ± 53 |
Arterial evaluation
Subjects were studied after resting supine for 20 min. The common carotid artery IMT was determined by high-resolution B-mode ultrasound imaging using a technique validated by Simons [6]. Adjustable gates were positioned at the junctions of the intima and media wall of the carotid artery, and the diameter was calculated and displayed in real time as the difference between the displacement waveforms of the anterior and posterior walls. Measurements were taken as the mean of five cardiac cycles. Stiffness parameter beta was calculated according to the formula: 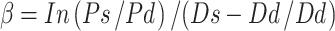 , where Ps and Pd represent the mean of three measurements of systolic and diastolic blood pressure, respectively, in the brachial artery as measured by a manual sphygmomanometer, and using Ds and Dd to represent the maximal and minimal diameters of the right common carotid artery measured by ultrasonic high resolution wall tracking (Aloka α-10, Japan; 7.5 MHz linear array probe).
, where Ps and Pd represent the mean of three measurements of systolic and diastolic blood pressure, respectively, in the brachial artery as measured by a manual sphygmomanometer, and using Ds and Dd to represent the maximal and minimal diameters of the right common carotid artery measured by ultrasonic high resolution wall tracking (Aloka α-10, Japan; 7.5 MHz linear array probe).
Echocardiographic methods
Immediately after assessing arterial stiffness, subjects were studied by conventional and tissue Doppler echocardiography (Aloka α-10, Tokyo, Japan) using a 1.5–2.5 MHz transducer. Standard views and standard techniques were employed according to the guidelines of the American Society of Echocardiography (ASE) [7].
M mode tracings from the parasternal long axis view were used to measure septal thickness, LV diameter, ejection fraction (EF), LV mass index (LVMI) and were calculated (method of Devereux with the application of the Penn convention).
Tissue Doppler measurements were made in four myocardial segments: longitudinal function from the velocities of basal septal and lateral segments, and basal anterior and basal inferior segments. Peak myocardial velocities in systole and early diastole were measured. Velocities of the four segments imaged were averaged. LV diastolic filling pressure was estimated from the ratio of mitral E wave, by pulsed wave Doppler to mitral annular early diastolic velocity (Em wave) by pulsed wave tissue Doppler [8].
Evaluation of central blood pressure
Radial artery pressure pulse waveform was recorded with an automated tonometric system, HEM-9000AI (Omron Healthcare, Kyoto, Japan) with subjects in a sitting position after at least 15 min of rest. The waveform was calibrated automatically using built-in oscillometric brachial sphygmomanometry. The peak and base of the radial pressure wave were adjusted to brachial systolic and diastolic blood pressure, respectively. The HEM-9000AI algorithm automatically performed online detection of central systolic blood pressure (cBP) and this was used as a parameter reflecting LV pressure load in univariate analysis of variance and multiple linear regression [9].
Blood biochemistry measurements
Overnight fasting serum and plasma samples were obtained for glucose, lipid, and other biochemical parameters. These biochemistry parameters were determined using standard laboratory techniques on a Cobas E601 (Roche, Germany).
Statistical analysis
Values are presented as means ± SD. In some cases a ratio is reported as well. Pearson’s correlation coefficients were calculated to estimate the association between variables. Independent determinants of β and E/Em ratio were analysed using stepwise multivariate linear regression. All statistical procedures were performed using SPSS statistical package, version 18.0 (SPSS Inc., Chicago, Illinois). Intercorrelated independent variables were removed from the regression model if multi co-linearity was detected by a tolerance < 0.10 or a variance inflation factor >10. Statistical significance was set at P < 0.05.
Results
Correlates of arterial stiffness and LV diastolic filling pressure
There was a direct relationship between β and LV global diastolic function (E/Em ratio) [correlation coefficients (r) = 0.255, p < 0.001] by univariate analysis as shown in Table 2. Beta positively related to body mass index, cBP, fasting blood glucose, and LV end-diastolic diameter (LVEDd).
Table 2.
Relationship between characteristic factor and stiffness parameter beta or LV filling pressure in subjects
| Variable | Stiffness parameter beta | E/Em ratio | ||
|---|---|---|---|---|
| r | p | r | p | |
| Body mass index (BMI) | 0.271 | <0.001 | 0.093 | 0.183 |
| Waist-to-hip ratio | 0.061 | 0.438 | 0.142 | 0.068 |
| Central arterial systolic blood pressure | 0.369 | <0.001 | 0.225 | 0.001 |
| High-density lipoprotein cholesterol | −0.080 | 0.430 | −0.085 | 0.461 |
| Low-density lipoprotein cholesterol | 0.121 | 0.085 | 0.096 | 0.071 |
| Triglycerides | 0.071 | 0.317 | 0.059 | 0.395 |
| Cholesterol | 0.122 | 0.085 | 0.103 | 0.068 |
| Fasting blood glucose | 0.138 | 0.047 | 0.231 | <0.001 |
| Creatinine | −0.073 | 0.332 | −0.261 | <0.001 |
| Uric acid | 0.086 | 0.222 | 0.061 | 0.381 |
| Stiffness parameter beta | — | — | 0.255 | <0.001 |
| LV ejection fraction | −0.053 | 0.488 | −0.366 | <0.001 |
| E/Em ratio | 0.255 | <0.001 | — | — |
| End-diastolic thickness of LV septum | 0.002 | 0.966 | 0.007 | 0.931 |
| LV end-diastolic diameter | 0.220 | 0.002 | 0.196 | 0.009 |
| LV mass index | 0.079 | 0.293 | 0.124 | 0.098 |
E/Em ratio was directly correlated with EF (P < 0.001) and LVEDd (P = 0.009). Similarly, E/Em ratio correlated with cBP and fasting blood glucose (Table 2). Notably, diastolic function directly correlated with creatinine whereas this parameter showed no correlation with arterial stiffness.
Multivariate analyses
Stepwise multiple regression analysis (parameters tested: age, gender, body mass index, smoking, cBP, HDL-C, LDL-C, fasting blood glucose) revealed that arterial stiffness was independently and directly correlated to age, smoking, and cBP. (R2 = 0.311, P < 0.05, Table 3).
Table 3.
Multivariate analysis of stiffness parameter beta and LV filling pressure
| Independent variable | Stiffness parameter beta | E/Em ratio | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.442 | <0.001 | 0.239 | <0.001 |
| Smoking | 0.236 | <0.001 | — | — |
| cBP | 0.321 | <0.001 | 0.419 | <0.001 |
| Fasting blood glucose | 0.150 | 0.022 | 0.477 | <0.001 |
| β | - | - | 0.180 | 0.004 |
| R2 | 0.311 | <0.05 | 0.381 | <0.01 |
Additionally, stepwise multiple regression analysis (parameters tested: age, gender, body mass index, smoking, cBP, HDL-C, LDL-C, fasting blood glucose and arterial stiffness) showed that E/Em ratio independently and directly correlated to age, cBP and arterial stiffness (R2 = 0.381, P < 0.01, Table 3).
Discussion
In the current study, the relationships between arterial characteristics and LV structural changes and function were evaluated in a cross-sectional survey of asymptomatic hypertensive adults with the following findings obtained: 1) The development of LV diastolic dysfunction is accompanied by enhanced arterial stiffness. Arterial and LV structural changes may be involved in the abnormal vascular-ventricular interaction; 2) LV dysfunction may have a direct relationship to arterial stiffening, with all subjects displaying similar risk factors; 3) Increased arterial stiffness, as an independent predictor of LV diastolic function, led to LV diastolic function.
The data presented show a positive correlation between arterial stiffness characteristics and LV filling pressure (E/Em ratio) and LVEDd. This is consistent with a possible mechanistic link between arterial stiffening and LV diastolic dysfunction. Eren indicated that aortic stiffness was increased in patients with hypertension, diabetes, or both after the exclusion of coronary artery disease, and that aortic stiffness and LV diastolic dysfunction are associated in these patients [10]. Abnormal arterial stiffness may potentially contribute to the development of LV diastolic dysfunction through increased pulse pressure and LV afterload, which may exacerbate sub-endocardial ischaemia, impair myocardial relaxation and promote interstitial fibrosis, leading to reduced LV compliance [11–13].
The univariate correlation analysis showed that LVEDd is correlated with both LV diastolic function and arterial stiffness. Changes in LV structure, especially LV diameter, may therefore control the progression from arterial stiffness to left ventricular diastolic dysfunction, which supports several published findings [13, 14]. Roman found that arterial stiffening was associated with concentric remodelling but not further hypertrophy of LV structure [15]. It has been proposed that abnormal LVEDd occurs earlier than LVM in the development of hypertension. Furthermore, there is some suggestion that LVEDd is more sensitive than LVMI in predicting LV diastolic dysfunction in the progression of hypertensive remodelling [16]. The current study suggests that subclinical atherosclerosis is associated with myocardial dysfunction, and that alterations in LV structure contribute to this dysfunction.
The multivariate model was adjusted for interacting factors such as age, gender, central systolic blood pressure, blood glucose and blood lipids. Of these, central systolic blood pressure, blood glucose and age were shown to be determinants of arterial stiffening, identically and independently influencing LV diastolic function.
Vascular-ventricular structural and functional changes occur with normal ageing [17]. However, the presence of cardiovascular risk factors accelerate their changes [18]. Stiffening in large elastic arteries is associated with multiple cardiovascular risk factors, including hypertension, dyslipidaemia, obesity, smoking, diabetes, and ageing, all of which also favour the development of atherosclerosis. These findings are supported by our current study [19, 20].
However, the effects of various risk factors on LV diastolic function were not entirely clear. Previous studies have also described an association between elevated blood pressure and incident heart failure in the absence of or independent from any coexistent coronary heart disease [21]. Conduit vessel resistance may increase myocardial vulnerability in coronary perfusion pressure and subendocardial ischaemia, particularly in the setting of coronary stenosis [22, 23].
The effect of statins on LV structure and function has also been reported in some studies [24, 25]. Mizuguchi reported statin therapy promotes LV diastolic function [25]. This could be mainly due to influence on inflammation rather than on the level of blood lipids. Additionally, fasting blood glucose increased with enhanced arterial stiffness and LV diastolic dysfunction, and insulin resistance in long-standing hypertension was related to LV mass, geometry and function [26–28]. Advanced glycation products have been reported to deteriorate arterial stiffness and LV diastolic function by crossing-linked, long-lived proteins, such as collagen and elastin, and altering cellular responses in the tissue [29].
In short, the similarity of histological changes in arterial wall and myocardium may contribute to the vascular-myocardium stiffening based on similar risk factors.
Undoubtedly there has been a great amount of study on the association of hypertension with both arterial stiffening and heart failure. A plausible explanation for the relationship between arterial stiffening and LV dysfunction invokes longstanding high blood pressure, chronic increasing haemodynamic loading on the LV, and subsequent myocardial fibrosis and stiffness as ultimately causing impaired function. However, we found that carotid arterial stiffness was associated with myocardial function even after adjustment for blood pressure. Therefore, it appeared from our study that arterial stiffness itself, and not just its causes or sequelaes, are directly associated with alterations in myocardial function. This finding coincides with recent work demonstrating that concurrent vascular and ventricular stiffening can progress even in the absence of cardiac hypertrophy [29].
Not surprisingly, previous studies have identified arterial stiffness itself as a risk factor for clinical heart failure [30]. However, we found arterial stiffness was related to subclinical myocardial dysfunction even after controlling for multiple risk factors and in the absence of clinical cardiovascular diseases.
There are several mechanisms that may also contribute to the relationship between arterial stiffening and myocardial dysfunction. Any decrease in diastolic blood pressure, as a result of arterial stiffness, may compromise coronary artery blood flow [31]. Impaired myocardial oxygen supply, particularly at the level of small coronary resistance vessels, may lead to fibrosis and the deposition of myocardial and vascular collagen [32]. Additionally, overactivity of the renin-angiotensin-aldosterone system, accompanied by enhanced arterial stiffness, produces both vascular and ventricular damage as well as pathological remodelling [33].
This study demonstrates a consistent association between arterial stiffness and impaired LV function in a cohort of asymptomatic individuals. By highlighting the presence of early vascular dysfunction in the setting of subclinical left ventricular dysfunction, this study provides evidence in support of a novel paradigm for understanding the pathogenesis of the progression of heart failure, especially HFPEF.
Because arterial stiffness may be modifiable, it should be a potential target for intervention of diastolic heart failure in future studies. Recently, it has been reported that exercise and administration of the angiotensin II receptor blockers could reduce vascular stiffness [34, 35]. Therefore, it could be concluded that exercise and effective medication therapy may directly improve LV diastolic function by increasing vascular elasticity. The results of our study may be important in considering diagnostic and therapeutic strategies aimed at HFPEF.
In summary, our findings suggest that LV dysfunction may be analogous with arterial stiffening, and that they both have similar risk factors. In addition, arterial stiffness is an independent predictor of LV diastolic function, suggesting that the severity of arterial stiffness is directly proportionate to the severity of LV dysfunction.
References
- 1.Senni M, Redfild MM. Heart failure with preserved systolic function: a different natural history? J Am Coll Cardiol. 2001;38:1277–1282. doi: 10.1016/S0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 2.Yambe M, Tomiyama H, Hirayama Y, et al. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res. 2004;27:625–631. doi: 10.1291/hypres.27.625. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.CIR.0000048123.22359.A0. [DOI] [PubMed] [Google Scholar]
- 4.Chow PC, Ho MH, Lee TL, et al. Relation of arterial stiffness to left ventricular structure and function in adolescents and young adults with pediatric-onset systemic lupus erythematosus. J Rheumatol. 2007;34:1345–1352. [PubMed] [Google Scholar]
- 5.Vinereanu D, Nicolaides E, Boden L, et al. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449–450. doi: 10.1136/heart.89.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons PC, Algra A, Bots ML, et al. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease) Circulation. 1999;100:951–957. doi: 10.1161/01.CIR.100.9.951. [DOI] [PubMed] [Google Scholar]
- 7.Waggoner AD, Bierig M. Tissue Doppler imaging: a useful echocardiographic method for the cardiac sonographer to assess systolic and diastolic ventricular function. J Am Soc Echocardiogr. 2001;14:1143–1152. doi: 10.1067/mje.2001.115391. [DOI] [PubMed] [Google Scholar]
- 8.Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of Arterial Stiffness to Left Ventricular Diastolic Function and Cardiovascular Risk Prediction in Patients >65 Years of Age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Agabiti-Rosei J, Mancia G, O’Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50(1):154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 10.Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43. doi: 10.1136/heart.90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alan S, Ulgen MS, Ozturk O, et al. Relation between coronary artery disease, risk factors and intima-media thickness of carotid artery, arterial distensibility and stiffness index. Angiology. 2003;54:261–267. doi: 10.1177/000331970305400301. [DOI] [PubMed] [Google Scholar]
- 12.Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Shigematsu Y, Hamada M, et al. Relative wall thickness is an independent predictor of left ventricular systolic and diastolic dysfunction in essential hypertension. Hypertens Res. 2001;24:493–499. doi: 10.1291/hypres.24.493. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Greco R, Mureddu G, et al. Relation of left ventricular diastolic properties to systolic function in arterial hypertension. Circulation. 2000;101:152–157. doi: 10.1161/01.CIR.101.2.152. [DOI] [PubMed] [Google Scholar]
- 15.Roman MJ, Ganau A, Saba PS, et al. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.HYP.36.4.489. [DOI] [PubMed] [Google Scholar]
- 16.Schillaci G, Mannarino MR, Pucci G, et al. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension. 2007;49:317–321. doi: 10.1161/01.HYP.0000255790.98391.9b. [DOI] [PubMed] [Google Scholar]
- 17.Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, Giannattasio C, Grassi G. Arterial distensibility in cardiovascular diseases. J Nephrol. 1998;11:284–288. [PubMed] [Google Scholar]
- 20.Sharrett AR, Ding J, Criqui MH, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Ohtsuka S, Kakihana M, et al. Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol. 1993;21:1497–1506. doi: 10.1016/0735-1097(93)90330-4. [DOI] [PubMed] [Google Scholar]
- 23.Kass DA, Saeki A, Tunin RS, et al. Adverse influence of systemic vascular stiffening on cardiac dysfunction and adaptation to acute coronary occlusion. Circulation. 1996;93:1533–1541. doi: 10.1161/01.CIR.93.8.1533. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara K, Shoji T, Kimoto E, et al. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005;12:205–210. doi: 10.5551/jat.12.205. [DOI] [PubMed] [Google Scholar]
- 25.Mizuguchi Y, Oishi Y, Miyoshi H, et al. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circ J. 2008;72:538–544. doi: 10.1253/circj.72.538. [DOI] [PubMed] [Google Scholar]
- 26.Vinereanu D, Nicolaides E, Tweddel AC, et al. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci. 2003;105(5):591–599. doi: 10.1042/CS20030168. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi H, Saitoh S, Takagi S, et al. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose:the Tanno and Sobetsu study. Diabetes. 2003;26:437–440. doi: 10.2337/diacare.26.2.437. [DOI] [PubMed] [Google Scholar]
- 28.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21(1):3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/A:1013749806227. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–138. doi: 10.1016/S0735-1097(00)00687-2. [DOI] [PubMed] [Google Scholar]
- 31.Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–564. doi: 10.1161/01.HYP.32.3.560. [DOI] [PubMed] [Google Scholar]
- 32.Strauer BE. Development of cardiac failure by coronary small vessel disease in hypertensive heart disease? J Hypertens Suppl. 1991;9:S11–S20. doi: 10.1097/00004872-199112002-00003. [DOI] [PubMed] [Google Scholar]
- 33.Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5:102–108. doi: 10.3317/jraas.2004.025. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 35.Agata J, Nagahara D, Kinoshita S, et al. Angiotensin II receptor blocker prevents increased arterial stiffness in patients with essential hypertension. Circ J. 2004;68:1194–1198. doi: 10.1253/circj.68.1194. [DOI] [PubMed] [Google Scholar]


