Abstract
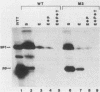
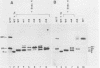
The effects of five single-amino-acid substitution mutations within the signal sequence of yeast prepro-alpha-factor were tested in yeast cells. After short pulse-labelings, virtually all of the alpha-factor precursor proteins from a wild-type gene were glycosylated and processed by signal peptidase. In contrast, the signal sequence mutations resulted in the accumulation of mostly unglycosylated prepro-alpha-factor after a short labeling interval, indicating a defect in translocation of the protein into the endoplasmic reticulum. Confirming this interpretation, unglycosylated mutant prepro-alpha-factor in cell extracts was sensitive to proteinase K and therefore in a cytosolic location. The signal sequence mutations reduced the rate of translocation into the endoplasmic reticulum by as much as 25-fold or more. In at least one case, mutant prepro-alpha-factor molecules were translocated almost entirely posttranslationally. Four of the five mutations also reduced the rate of proteolytic processing by signal peptidase in vivo, even though the signal peptide alterations are not located near the cleavage site. This study demonstrates that a single-amino-acid substitution mutation within a eucaryotic signal peptide can affect both translocation and proteolytic processing in vivo and may indicate that the recognition sequences for translocation and processing overlap within the signal peptide.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Young E. T. Single-amino-acid substitutions within the signal sequence of yeast prepro-alpha-factor affect membrane translocation. Mol Cell Biol. 1988 May;8(5):1915–1922. doi: 10.1128/mcb.8.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. K., Bentivoglio G. P., Lively M. O. Partial purification of microsomal signal peptidase from hen oviduct. J Cell Biochem. 1986;32(3):193–200. doi: 10.1002/jcb.240320305. [DOI] [PubMed] [Google Scholar]
- Bedwell D. M., Strobel S. A., Yun K., Jongeward G. D., Emr S. D. Sequence and structural requirements of a mitochondrial protein import signal defined by saturation cassette mutagenesis. Mol Cell Biol. 1989 Mar;9(3):1014–1025. doi: 10.1128/mcb.9.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Stevens T. H. Yeast carboxypeptidase Y can be translocated and glycosylated without its amino-terminal signal sequence. J Cell Biol. 1987 May;104(5):1183–1191. doi: 10.1083/jcb.104.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. S., Cornell D. G., Dluhy R. A., Gierasch L. M. Conformations of signal peptides induced by lipids suggest initial steps in protein export. Science. 1986 Jul 11;233(4760):206–208. doi: 10.1126/science.2941862. [DOI] [PubMed] [Google Scholar]
- Burkholder A. C., Hartwell L. H. The yeast alpha-factor receptor: structural properties deduced from the sequence of the STE2 gene. Nucleic Acids Res. 1985 Dec 9;13(23):8463–8475. doi: 10.1093/nar/13.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C., Tarentino A. L., Maley F., Atkinson P. H., Trimble R. B. Glycoprotein synthesis in yeast. Identification of Man8GlcNAc2 as an essential intermediate in oligosaccharide processing. J Biol Chem. 1982 Dec 25;257(24):14657–14666. [PubMed] [Google Scholar]
- Böhni P. C., Deshaies R. J., Schekman R. W. SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol. 1988 Apr;106(4):1035–1042. doi: 10.1083/jcb.106.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Garcia P. D., Walter P. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell. 1986 May 9;45(3):397–406. doi: 10.1016/0092-8674(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Hansen W., Walter P. Prepro-carboxypeptidase Y and a truncated form of pre-invertase, but not full-length pre-invertase, can be posttranslationally translocated across microsomal vesicle membranes from Saccharomyces cerevisiae. J Cell Biol. 1988 Apr;106(4):1075–1081. doi: 10.1083/jcb.106.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Allison D. S., Müller U., Schatz G. Amino-terminal deletions in the presequence of an imported mitochondrial protein block the targeting function and proteolytic cleavage of the presequence at the carboxy terminus. J Biol Chem. 1987 Jan 25;262(3):1420–1424. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Sauer R. T., Botstein D. Diverse effects of mutations in the signal sequence on the secretion of beta-lactamase in Salmonella typhimurium. Cell. 1982 Oct;30(3):903–914. doi: 10.1016/0092-8674(82)90295-1. [DOI] [PubMed] [Google Scholar]
- Kurjan J. Alpha-factor structural gene mutations in Saccharomyces cerevisiae: effects on alpha-factor production and mating. Mol Cell Biol. 1985 Apr;5(4):787–796. doi: 10.1128/mcb.5.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Hall B. D. Mutations at the Saccharomyces cerevisiae SUP4 tRNA(Tyr) locus: isolation, genetic fine-structure mapping, and correlation with physical structure. Mol Cell Biol. 1982 Dec;2(12):1501–1513. doi: 10.1128/mcb.2.12.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Wu H. C. Purification and characterization of the outer membrane lipoprotein from an Escherichia coli mutant altered in the signal sequence of prolipoprotein. J Biol Chem. 1980 Feb 10;255(3):1160–1163. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: translocation and glycosylation of prepro-alpha-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J. 1986 May;5(5):1031–1036. doi: 10.1002/j.1460-2075.1986.tb04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Toyn J., Hibbs A. R., Sanz P., Crowe J., Meyer D. I. In vivo and in vitro analysis of ptl1, a yeast ts mutant with a membrane-associated defect in protein translocation. EMBO J. 1988 Dec 20;7(13):4347–4353. doi: 10.1002/j.1460-2075.1988.tb03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Evans E. A., Blobel G. Prepro-alpha-factor has a cleavable signal sequence. J Biol Chem. 1988 May 5;263(13):6209–6214. [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Hartmann E., Rapoport T. A. A signal sequence receptor in the endoplasmic reticulum membrane. 1987 Aug 27-Sep 2Nature. 328(6133):830–833. doi: 10.1038/328830a0. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]