Abstract
IgE antibody-mediated allergies affect more than 25% of the population worldwide. To investigate therapeutic and preventive effects of passive immunization with allergen-specific IgG antibodies on allergy in mouse models we used clinically relevant pollen allergens. In a treatment model, mice were sensitized to the major birch pollen allergen Bet v 1 and to the major grass pollen allergens, Phl p 1 and Phl p 5 and then received passive immunization with rabbit IgG antibodies specific for the sensitizing or an unrelated allergen. In a prevention model, mice obtained passive immunization with allergen-specific rabbit IgG before sensitization. Kinetics of the levels of administered IgG antibodies, effects of administered allergen-specific IgG on allergen-specific IgE reactivity, the development of IgE and IgG responses and on immediate allergic reactions were studied by ELISA, rat basophil leukaemia degranulation assays and skin testing, respectively. Treated mice showed an approximately 80% reduction of allergen-specific IgE binding and basophil degranulation which was associated with the levels of administered allergen-specific IgG antibodies. Preventive administration of allergen-specific IgG antibodies suppressed the development of allergen-specific IgE and IgG1 antibody responses as well as allergen-induced basophil degranulation and skin reactivity. Our results show that passive immunization with allergen-specific IgG antibodies is effective for treatment and prevention of allergy to clinically important pollen allergens in a mouse model and thus may pave the road for the clinical application of allergen-specific antibodies in humans.
Abbreviations: i.n., intranasal; SIT, allergen-specific immunotherapy
Keywords: Allergy, Prevention, Treatment, Antibodies, Mouse, Passive immunization
Introduction
IgE-mediated allergy affects more than 25% of the population worldwide and shows a continuously increasing prevalence (Floistrup et al. 2006). Allergen-specific immunotherapy (SIT) is currently the only antigen-specific, disease-modifying treatment of allergy and has long-lasting effects (Shamji and Durham 2011). Since the first reports indicated that SIT induces allergen-specific IgG antibodies which block IgE-mediated inflammation (Cooke et al. 1935; Loveless 1940), it has been shown that SIT-induced allergen-specific IgG also reduces T cell reactivity and boosts of IgE production (Larche et al. 2006). Passive immunization of patients with allergen-specific IgG antibodies has been successfully used so far only in combination with active SIT mainly to reduce the sensitivity of patients but not yet as sole treatment procedure (Bernstein et al. 1979; Bousquet et al. 1987; Muller et al. 1986). It has been also reported that SIT-induced allergen-specific IgG antibodies cross the placenta (Flicker et al. 2009) and may protect the offspring from sensitization (Glovsky et al. 1991). Furthermore, it has been demonstrated that elevated cord blood levels of allergen-specific IgG are associated with less allergy development in children (Jenmalm and Bjorksten 2000).
Experimental studies performed in mice and rats with model antigens such as ovalbumin suggested that prenatal active immunization induces allergen-specific IgG antibodies which protect against allergen-induced sensitization and allergic inflammation in the off-spring (Fusaro et al. 2007; Jarrett and Hall 1983; Polte and Hansen 2008; Polte et al. 2008; Uthoff et al. 2003). There are also experimental animal studies using ovalbumin or goat-anti IgD as immunogens demonstrating that passive administration of specific IgG antibodies may be effective in suppressing allergic sensitization, allergic inflammation and even anaphylactic shock (Moerch et al. 2006; Sehra et al. 2003; Strait et al. 2006; Uthoff et al. 2003).
However, so far no studies have been performed with allergens which are relevant for allergy in humans. Here we used clinically important grass pollen allergens (i.e., Phl p 1, Phl p 5) (Laffer et al. 1994; Vrtala et al. 1993; Westritschnig et al. 2008), the major birch pollen allergen Bet v 1 (Breiteneder et al. 1989; Niederberger et al. 1998a,b; Pauli et al. 2008) and corresponding allergen-specific IgG antibodies to investigate whether passive immunization with allergen-specific IgG antibodies can suppress IgE-mediated allergic reactions in a mouse model for treatment. Furthermore, we studied in a preventive mouse model if passive immunization can suppress allergic sensitization.
Materials and methods
Allergens, animals, rabbit immune sera
Purified recombinant allergens (rBet v 1, rPhl p 1, rPhl p 5) were obtained from BIOMAY (Vienna, Austria). Female 6–8 week-old BALB/c mice were purchased from Charles River (Sulzfeld, Germany) and kept in the animal care unit of the Department of Pathophysiology and Allergy Research, Medical University of Vienna according to the local guidelines for animal care. All animal experiments were approved by the Animal Ethics Committee of the Medical University of Vienna and the Austrian Federal Ministry of Science and Research (66009/186-II/10b/2008). Pollen allergen-specific rabbit IgG antibodies were obtained by immunization of rabbits with purified recombinant allergens (rBet v 1, rPhl p 1, rPhl p 5) using complete and incomplete Freund’ s adjuvant (CFA, IFA), respectively (Charles River, Kislegg, Germany).
Sensitization and passive antibody treatment of mice
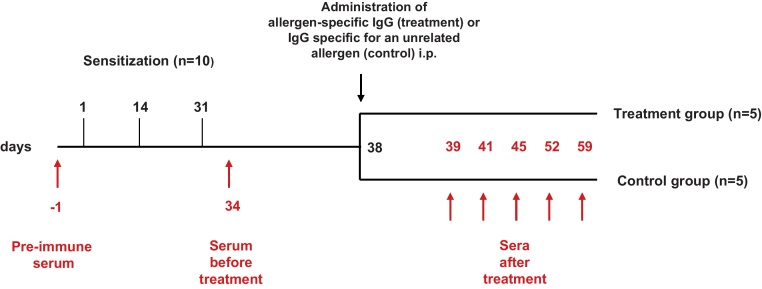
For the therapy model, mice were sensitized by three subcutaneous (s.c.) immunizations (days 1, 14 and 31) with each of the purified recombinant allergens (5 μg/mouse) adsorbed to aluminium hydroxide (Al(OH)3) (Alu-Gel-S; Serva, Ingelheim, Germany) (Fig. 1). Groups of ten mice each were sensitized to rBet v 1, rPhl p 1 or rPhl p 5. Blood samples were taken from the tail veins before sensitization, at day 34 and at days 39, 41, 45, 52 and 59 (Fig. 1). Allergic sensitization was confirmed by the measurement of allergen-specific IgE antibodies at day 34. Mice were then randomized into groups of 5 mice so that mice of each group had comparable allergen-specific IgE levels. Mice in the treatment groups received one intraperitoneal (i.p.) injection of 0.5 ml allergen-specific rabbit IgG whereas mice from the control groups received one i.p. injection of rabbit IgG specific for an unrelated allergen (Fig. 1).
Fig. 1.
Scheme of treatment by passive immunization with allergen-specific IgG antibodies. Groups of 10 mice were sensitized three times (days 1, 14 and 31) with aluminium hydroxide-adsorbed allergen and then randomized into two groups (n = 5) receiving allergen-specific IgG or IgG specific for an unrelated allergen. Sera were obtained at the time points indicated.
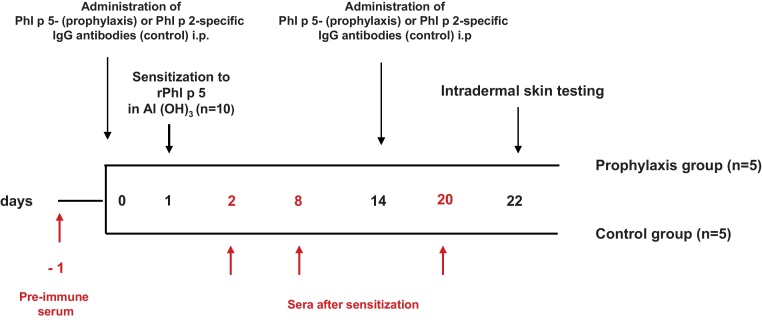
For the preventive model, groups of mice (n = 5) received either i.p. 0.5 ml Phl p 5-specific rabbit IgG antibodies (prophylaxis group) or 0.5 ml rabbit IgG antibodies specific for an unrelated allergen, Phl p 2 (control group) on day 0 and, on day 14 (Fig. 2). On day 1 mice of both groups were simultaneously sensitized s.c. with 5 μg rPhl p 5 and 5 μg rBet v 1 adsorbed to aluminium hydroxide (Al(OH)3). Blood was collected from the tail veins before sensitization as well as on days 2, 8 and 20 (Fig. 2).
Fig. 2.
Scheme for prophylactic administration of allergen-specific IgG antibodies. Mice received Phl p 5-specific IgG (prophylaxis group) or Phl p 2-specific IgG (control group) and were then sensitized to Phl p 5. Bleeding, administration of antibodies and intradermal testing were done at the time points indicated.
Measurement of total IgE levels, allergen-specific IgE and IgG1 antibodies as well as of allergen-specific rabbit IgG in mouse sera
Total IgE antibody levels were determined in mice using an IgE ELISA Set (Becton Dickinson, New Jersey, US). Optical densities corresponding to bound IgE antibodies (OD at 405 nm, reference OD at 490 nm) were determined with an ELISA reader (Spectramax PLUS, Molecular Devices, Sunnyvale, CA). Results are means of duplicate determinations with variations of less than 10%. To determine the IgE concentrations, mean OD values of the tested sera were compared to the mean OD values of the standard curve and calculated IgE titres were multiplied by the dilution factor of the tested sera and expressed in ng/ml.
Allergen-specific mouse IgE and IgG1 antibodies were determined by ELISA (Linhart et al. 2007). Optical densities corresponding to bound antibodies (OD at 405 nm, reference OD at 490 nm) were determined with an ELISA reader (Spectramax PLUS, Molecular Devices, Sunnyvale, CA). Results represented means of duplicate determinations with variations of less than 10%. The percentage inhibition of IgE binding by rabbit IgG was calculated as follows: Percentage inhibition = 100 − ODserum after treatment × 100/OD serum before treatment.
Allergen-specific rabbit IgG in sera from mice were measured by ELISA. ELISA plates (Nunc, Maxisorp) were coated with 100 μl (c = 1 μg/ml) rBet v 1, rPhl p 1, or rPhl p 5 in 0.1 M bicarbonate buffer, pH 9.6 at 4 °C overnight. Plates were washed twice with TBST containing 0.5% (w/v) BSA and blocked with TBST containing 3% (w/v) BSA at 37 °C for 3 h. After blocking, plates were incubated with sera (duplicates) taken before and after antibody treatment (diluted 1:1000, 1:5000, 1:10,000; 1:50,000 in TBST/0.5% (w/v) BSA) overnight at 4 °C. After washing 5 times with TBST/0.5% w/v BSA, rabbit IgG antibodies were detected with HRP-labelled donkey anti-rabbit IgG antibodies (GE Healthcare, Little Chalfont Buckinghamshire, UK) diluted 1:1000 in TBST/0.5% (w/v) BSA. HRP-labelled donkey anti-rabbit IgG antibodies showed no reactivity with mouse sera obtained before treatment ensuring specificity for rabbit IgG. Results represent means of duplicate determinations with variations of less than 10%. Allergen-specific rabbit IgG levels determined in sera 72 h, 1 week and 2 weeks after treatment were expressed as percentages of allergen-specific IgG levels measured 24 h after treatment.
Degranulation experiments with rat basophil leukaemia cells
The rat basophil leukaemia cell subline RBL-2H3 was maintained in RPMI medium (Biochrom, AG, Berlin, Germany) supplemented with 10% FCS (PAA, Pasching, Austria). RBL-2H3 cells were plated in 96-well tissue-culture plates (4–6 × 105 ml; Nunc, Roskilde, Denmark) and incubated at 37 °C in 5% CO2 overnight. The cell layer was washed twice with 1× Tyrode buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 0.5 mmol/L MgCl2, 1.8 mmol/L CaCl2, 0.4 mmol/L NaH2PO4, 5.6 mmol/L d-glucose, and 0.1% (w/v) BSA, pH 7.2; Sigma–Aldrich, Vienna, Austria).
The therapeutic effect of administered allergen-specific rabbit IgG was determined by diluting sera obtained from mice before and after antibody treatment 1:10 in RPMI, addition of different doses of allergens (0.02 μg/ml to 0.5 μg/ml) for 10 min to form immune complexes and exposing these complexes for 3 h to RBL cells and subsequent measurement of β-hexosaminidase release. β-Hexosaminidase was measured using 80 μmol/L 4-methyl-umbelliferyl- N-acetyl-β-d-glucosamine (Sigma–Aldrich) in citrate buffer (0.1 mol/L, pH 4.5) for 1 h at 37 °C. The reaction was stopped by the addition of glycine buffer (0.2 mol/L glycine, 0.2 mol/L NaCl, pH 10.7), and the fluorescence was measured at an extinction wavelength of 360 nm to the emission wavelength of 465 nm by using a fluorescence microplate reader (Perkin Elmer, Wallac, Vienna, Austria). β-Hexosaminidase release was expressed as a percentage of the total β-hexosaminidase release that was obtained by lysing the cells by addition of 1% Triton-X-100 (Merck, Darmstadt, Germany). Determinations were done as triplicates and are displayed as mean values ± SDs after subtraction of background. Background release was determined by loading cells with sera without addition of allergen.
The effects of preventive administration of allergen-specific IgG were studied by incubating RBL cells with sera (diluted 1:20 in RPMI medium) for 2 h. Then cells were washed two times with 1× Tyrode buffer to remove unbound antibodies. Cross-linking of the FcɛRI-bound IgE was induced by adding different doses of allergens (0.02 μg/ml to 0.5 μg/ml) for 1 h. β-Hexosaminidase release of RBL cells was measured as described above.
In vivo skin testing
Intradermal skin tests were performed in mice that had been treated prophylactically with allergen-specific antibodies before they were sensitized. One hundred microlitres of 0.5% Evans blue (Sigma) were injected intravenously (i.v.) into the tail veins of the mice. Subsequently 30 μl of rPhl p 5 (0.5 μg/ml) and rBet v 1 (0.5 μg/ml) were injected intradermally into the shaved abdominal skin. The mast cell degranulation compound 48/80 (20 μg/ml; Sigma) was used as a positive control and 1× PBS was injected as a negative control. After 20 min mice were sacrificed and skinned. The blue staining on the inverted skin was documented by photography.
Results
Passive immunization of mice with allergen-specific IgG antibodies inhibits allergen-specific IgE binding
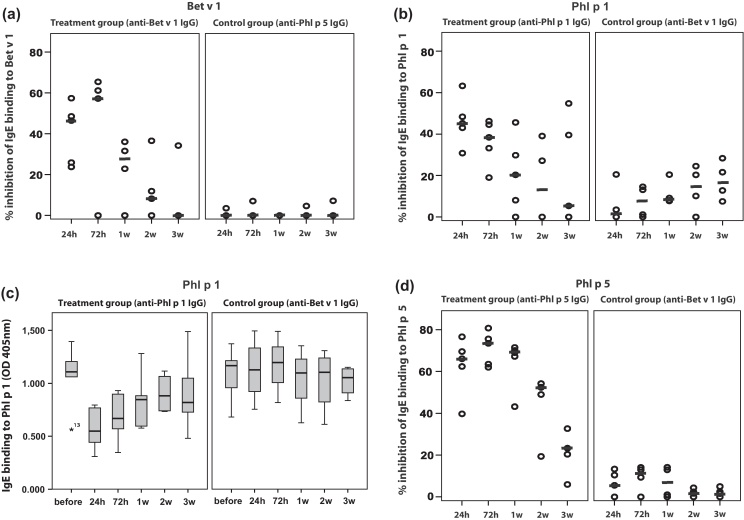
Fig. 3A–C shows the effects of passive immunization with allergen-specific antibodies on allergen-specific IgE binding. Sera from mice that had received Bet v 1-specific IgG antibodies showed a reduction of IgE binding to Bet v 1 between 23.8% and 57.4% (median: 46.5%) 24 h after antibody treatment (Fig. 3A, left panel). One week after antibody treatment still 27.2% inhibition of Bet v 1-specific IgE binding was observed which decreased during the next 2 weeks to 0%. No inhibition of IgE binding to Bet v 1 was observed in mice which had been immunized with IgG antibodies specific for an unrelated allergen (i.e., Phl p 5) (Fig. 3A, right panel).
Fig. 3.
Changes of specific IgE binding to allergens ((A) Bet v 1; (B and C) Phl p 1; (D) Phl p 5) in treated mouse groups. The percentages inhibition of allergen-specific IgE binding are shown for the individual mice at different points of time (x-axes: 24 h, 72 h, 1 week, 2 week, 3 week) after treatment with allergen-specific IgG antibodies (left panels) or with IgG antibodies specific for an unrelated allergen (right panels) (y-axes). Medians are indicated by dashes. In (C) the OD values corresponding to the levels of allergen-specific IgE are shown as box plots with indicated median values (y-axis) over time (x-axis) for the anti-Phl p 1-treated group and an anti-Bet v 1-treated control group shown in (B).
Similar results were obtained for Phl p 1- and Phl p 5-sensitized mice (Fig. 3B–D). Phl p 1-sensitized mice which were treated with Phl p 1-specific IgG antibodies showed a median reduction of IgE binding to Phl p 1 of 45.2% (ranging from 30.8 to 63.3%) 24 h after treatment and of 20.3% after 1 week (Fig. 3B, left panel). Fig. 3C shows the reduction of the Phl p 1-specific IgE levels for the latter group of mice and a control group treated with anti-Bet v 1 IgG. No relevant inhibition of IgE binding to Phl p 1 was observed in Phl p 1-sensitized mice which had received Bet v 1-specific IgG antibodies (Fig. 3B and C). The reduction of IgE binding to Phl p 5 in Phl p 5-sensitized mice treated with Phl p 5-specific IgG varied between 39.7% and 76.6% (median: 66.1%) 24 h after treatment, remained at around 69% after 1 week and even after 3 weeks was in the range of 23.3% (Fig. 3D, left panel). No relevant inhibition of IgE binding to Phl p 5 was found for mice which had been immunized with Bet v 1-specific IgG (Fig. 3D, right panel).
To investigate if fluctuations of total IgE levels affect the measurement of allergen-specific IgE binding total IgE levels were measured with a sandwich ELISA but were found to show no relevant alterations over the treatment period (data not shown).
Kinetics of the levels of administered allergen-specific IgG antibodies
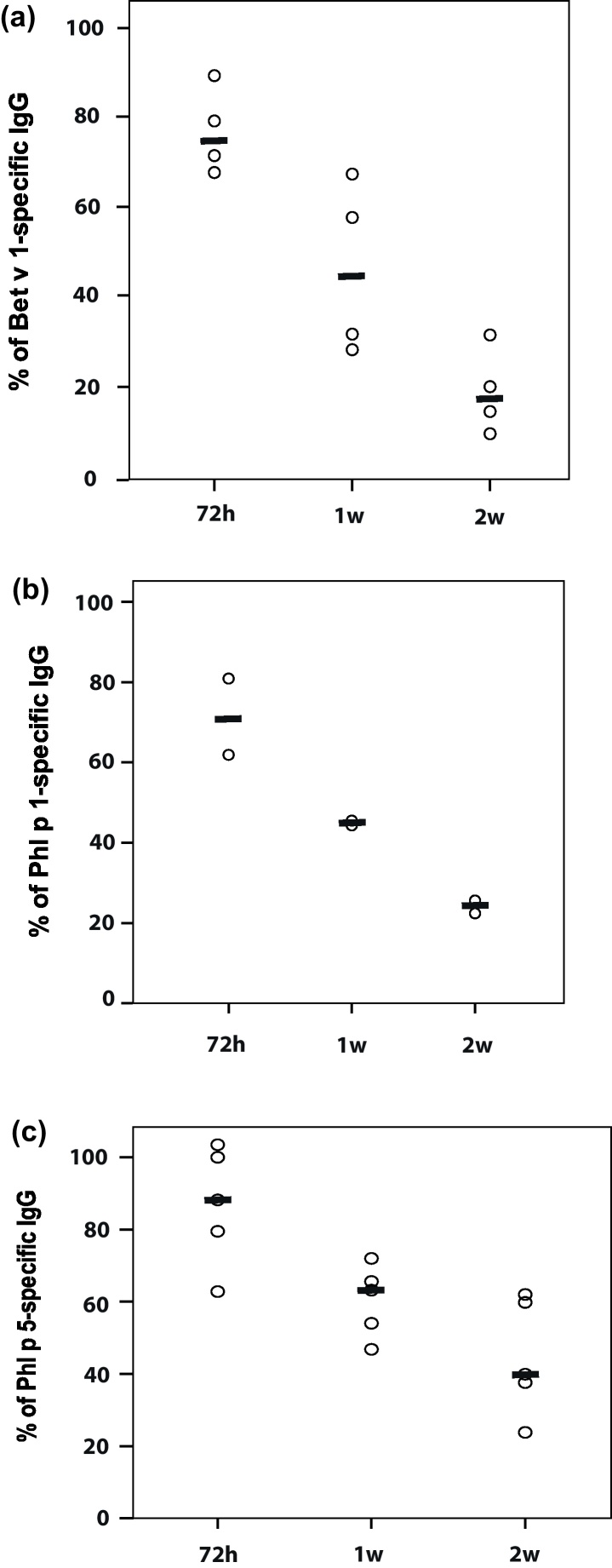
To assess the kinetics of the levels of administered allergen-specific IgG antibodies we compared the levels of allergen-specific rabbit IgG in mouse sera at different time points after immunization. The levels of allergen-specific IgG antibodies measured in the blood of the mice 24 h after immunization were compared with the levels measured at 72 h, 1 week and 2 weeks. 72 h after antibody injection approximately 75% (Bet v 1), 71% (Phl p 1) or 88% (Phl p 5) of allergen-specific IgG reactivity as compared to the 24 h level were found (Fig. 4A–C). Even after 2 weeks approximately 20% of the administered IgG antibodies could be detected for the Bet v 1 and Phl p 1-specific IgG antibodies (i.e., Bet v 1: median 17.8%; Phl p 1: median 24%) and almost 40% of the Phl p 5-specific IgG (i.e., median 39.9%). Due to the fact that the titres of allergen-specific IgG were higher in the anti-Phl p 5 antiserum compared to the anti-Bet v 1 and anti-Phl p 1 antisera the decline of allergen-specific rabbit IgG was slower for the anti-Phl p 5-treated animals.
Fig. 4.
Time-dependent decline of applied (A) Bet v 1- (B) Phl p 1- or (C) Phl p 5-specific IgG antibodies. Allergen-specific IgG binding of sera taken after treatment (72 h, 1week, 2week) is expressed as percentage of allergen-specific IgG binding of sera taken 24 h after treatment and displayed on the y-axes. Medians are indicated by dashes.
Passively applied allergen-specific IgG antibodies block immediate allergic reactions in sensitized mice
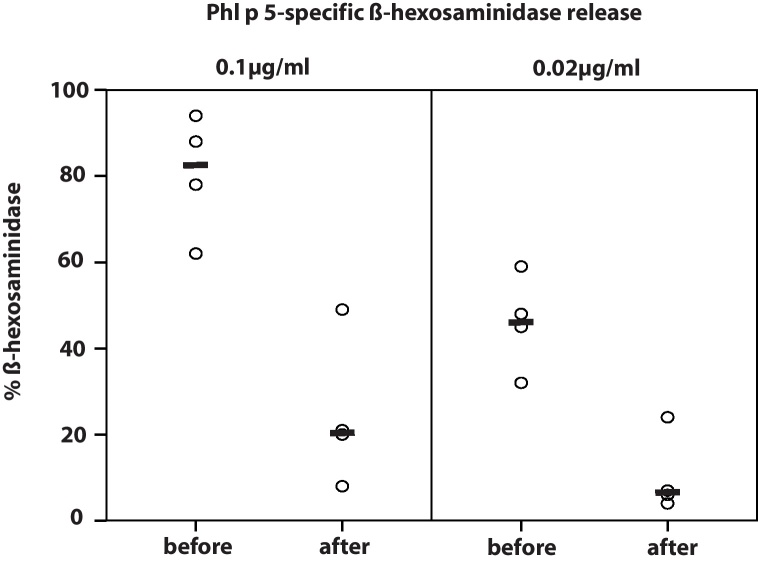
The therapeutic potential of passively applied allergen-specific IgG antibodies was studied in basophil cell degranulation experiments. Sera from passively immunized mice were incubated with allergens, exposed to RBL-2H3 cells and the release of β-hexosaminidase was measured. When sera were taken from Phl p 5-sensitized mice shortly before administration of Phl p 5-specific IgG antibodies a median β-hexosaminidase release of 45% was found for a concentration of 0.02 μg/ml Phl p 5 and of 78% for 0.1 μg/ml. When sera were taken from the same mice 24 h after passive immunization a strong reduction of the RBL degranulation was observed (6% median degranulation at 0.02 μg/ml Phl p 5 and 20% median degranulation at 0.1 μg/ml Phl p 5) (Fig. 5).
Fig. 5.
Inhibition of basophil degranulation by allergen-specific IgG antibodies. Two concentrations of Phl p 5 (0.02 μg/ml; 0.1 μg/ml; x-axis) were pre-incubated with sera taken before (before) and after antibody treatment (after) and exposed to RBL cells. The percentages of total β-hexosaminidase release are displayed on the y-axis. Medians are indicated by dashes.
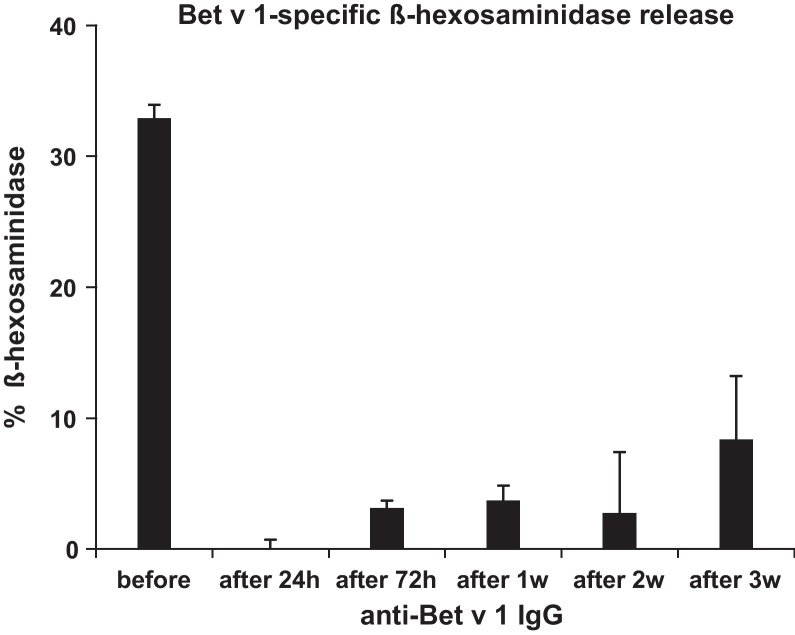
We also investigated the duration of the inhibitory effect of allergen-specific IgG in a Bet v 1-sensitized mouse. Serum samples were taken before (before) and 24 h, 72 h, 1 week, 2 weeks and 3 weeks after treatment, incubated with Bet v 1 and then exposed to RBL cells. Before the administration of Bet v 1-specific IgG, Bet v 1 induced a β-hexosaminidase release of approximately 35% which was fully suppressed at 24 h and remained reduced to approximately 10% even after 3 weeks of treatment (Fig. 6).
Fig. 6.
Inhibition of basophil degranulation by administered allergen-specific IgG antibodies. Bet v 1 was pre-incubated with serum obtained before (before) or after (24 h, 72 h, 1 week, 2 week, 3 week) treatment with Bet v 1-specific IgG. Complexes were exposed to RBL cells. β-Hexosaminidase release (means of triplicates) is expressed as percentage of total β-hexosaminidase release (y-axis).
Prophylactic application of allergen-specific IgG antibodies prevents the development of allergen-specific IgE and IgG1 antibodies and allergic sensitization
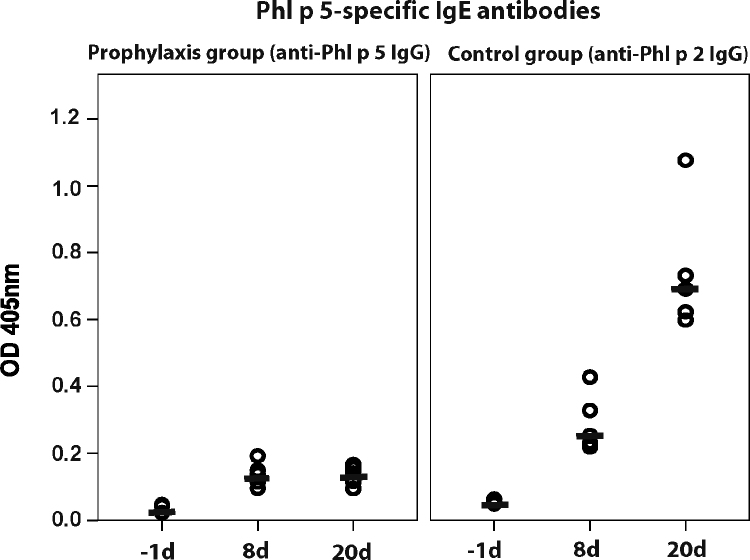
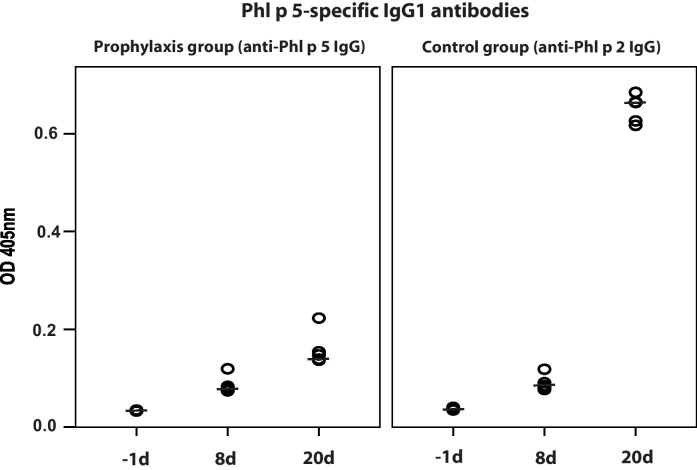
To evaluate whether prophylactic antibody treatment influences the development of allergen-specific IgE and IgG1 responses, mice received Phl p 5-specific rabbit IgG antibodies one day prior to sensitization with Phl p 5 (Fig. 2). Sera from mice treated with Phl p 5-specific IgG antibodies showed no relevant IgE and IgG1 binding to Phl p 5 (Fig. 7, left panel and Fig. 8, left panel) whereas sera of mice which had received antibodies with specificity to an unrelated allergen, Phl p 2 displayed robust IgE and IgG1 binding to rPhl p 5 (Fig. 7, right panel and Fig. 8, right panel). The development of Bet v 1-specific IgE and IgG1 antibodies was neither affected in mice receiving Phl p 5-specific nor in mice receiving Phl p 2-specific IgG antibodies (data not shown).
Fig. 7.
Development of Phl p 5-specific IgE antibodies in mice after prophylactic administration of Phl p 5-specific IgG antibodies (left panel) or Phl p 2-specific IgG antibodies (right panel) at different points of time (x-axes; −1day, 8day, 20day). OD levels (means of duplicates) (y-axes) correspond to bound IgE antibodies. Medians are indicated by dashes.
Fig. 8.
Development of Phl p 5-specific IgG1 antibodies in mice after prophylactic administration of Phl p 5-specific IgG antibodies (left panel) or Phl p 2-specific IgG antibodies (right panel) at different points of time (x-axes; −1day, 8day, 20day). OD levels (means of duplicates) (y-axes) correspond to bound IgG1 antibodies. Medians are indicated by dashes.
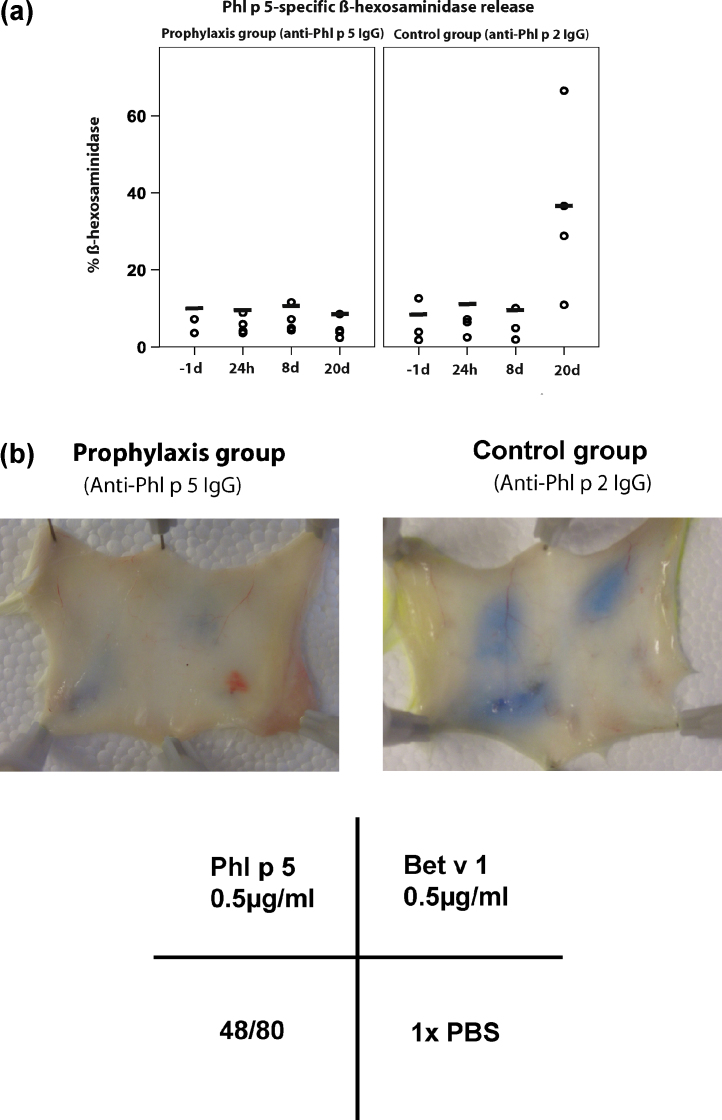
In order to study whether the lack of allergen-specific IgE binding is due to the presence of the administered allergen-specific IgG antibodies which compete with IgE for the binding to the allergen or due to the suppression of the development of allergic sensitization we performed basophil degranulation experiments. RBL cells bound only mouse IgE but not the administered competing allergen-specific rabbit IgG (data not shown). Fig. 9A, left panel shows that RBL cells exposed to sera from mice which had received prophylactic immunization with Phl p 5-specific IgG induced no relevant Phl p 5-specific degranulation even 20 days after treatment. This effect was found to last even for 40 days despite a second sensitization given at day 22 after the first sensitization (data not shown). By contrast, mice which had received a prophylactic immunization with unrelated (i.e., Phl p 2-specific) IgG showed Phl p 5-specific basophil degranulation (Fig. 9A, right panel).
Fig. 9.
(A) Effects of the application of allergen-specific IgG antibodies on basophil degranulation. Sera from mice obtained before (−1day) or after administration of Phl p 5-specific IgG (left panel) or Phl p 2-specific IgG (right panel) (24 h, 8day, 20day) were exposed to RBL cells. Then cells were washed and exposed to Phl p 5. β-Hexosaminidase releases (dashes indicate medians) are expressed as percentage of total releases (y-axes). (B) Lack of Phl p 5-specific immediate skin reactions in mice treated with Phl p 5-specific IgG. Evans blue extravasation in a representative mouse treated with Phl p 5-specific (left panel) or in mouse treated with Phl p 2-specific IgG (right panel) after intra-dermal injection of Phl p 5, Bet v 1, 48/80 or PBS.
The in vivo relevance of the prophylactic immunization was shown by in vivo skin testing. No Phl p 5-specific skin reaction could be induced in mice which had received Phl p 5-specific IgG antibodies before sensitization whereas reactions to Bet v 1 and compound 48/80 were not affected (Fig. 9B, left panel). By contrast, Phl p 5-specific skin reactions were elicited in mice which had been treated with Phl p 2-specific IgG antibodies (Fig. 9B, right panel).
Discussion
In this study we demonstrated in mouse models of allergic sensitization to clinically relevant pollen allergens that passive immunization with allergen-specific IgG antibodies is effective for treatment and prevention of allergy. In a model for treatment we used three important seasonal pollen allergens, Bet v 1, the major birch pollen allergen, as well as Phl p 1 and Phl p 5, two major grass pollen allergens (Niederberger et al. 1998a; Niederberger et al. 1998b; Westritschnig et al. 2008) for sensitization and highly specific rabbit IgG antibodies which were obtained by immunization with the purified recombinant allergens for treatment.
In order to mimic the treatment of established allergy, mice were sensitized first and then received passive immunization with allergen-specific IgG antibodies. Single passive administration inhibited allergen-specific IgE binding and allergen-induced basophil degranulation for a period of up to 3 weeks.
Similar observations were made earlier in mouse models based on the model antigen ovalbumin where it was shown that intranasally (i.n.) administered OVA-specific IgG antibodies reduced signs of allergic asthma (Moerch et al. 2006; Sehra et al. 2003). The authors proposed OVA interception through IgG and FcγR binding on alveolar macrophages followed by an increase of INF-γ-secreting T cells as responsible mechanism for this inhibition (Sehra et al. 2003). By contrast, Strait et al. suggested that the protective effect of allergen-specific IgG may be mediated by two mechanisms, one being capture of antigen before it can cross-link FcɛRI-bound IgE and the other being cross-linking of FcɛRI to the inhibitory FcγRIIb through the allergen/IgG/IgE complex (Strait et al. 2006). In our models the allergen-specific IgG was in excess to IgE because after administration of IgG almost no IgE binding to allergens could be detected. It is therefore most likely that the inhibition of basophil degranulation and the inhibition of IgE sensitization observed in our models were due to capture of allergen. We think that passive immunization could be a possible treatment option especially for seasonal allergies (e.g., pollen allergies) which may be treated by one pre-seasonal and eventually an additional maintenance immunization depending on the length of the pollen season. In fact, it has been demonstrated that human allergen-specific IgG antibodies can be identified for such a purpose (Visco et al. 1996; Flicker et al. 2002; Padavattan et al. 2009). These antibodies can be modified to increase affinity, in vivo half-life and mechanisms of action through modification of their constant domains (e.g., mutation of CDRs, change of glycosylation, ability to bind to Fcγ-receptors) (reviewed in Flicker et al. 2011).
In a second set of our experiments we investigated whether prophylactic administration of allergen-specific IgG antibodies in naïve mice can prevent allergic sensitization. Interestingly, we found that mice having received allergen-specific IgG antibodies neither developed allergen-specific IgE nor allergen-specific IgG responses and failed to exhibit any allergic reactions. This suppression was allergen-specific because the IgE and IgG responses against another unrelated allergen were not inhibited. We consider the latter finding interesting because it is in agreement with data showing that allergen-specific IgG antibodies which are transmitted from SIT-treated mothers to their children may protect against allergic sensitization (Glovsky et al. 1991). It has also been reported that children with high allergen-specific IgG levels in cord blood develop less frequently allergies (Jenmalm and Bjorksten 2000). Furthermore, data from experimental animal models suggest that pre-conceptional immunization with allergens or passive immunization with allergen-specific IgG leads to transmission of allergen-specific IgG to the off-spring and prevents allergic sensitization (Fusaro et al. 2007; Polte and Hansen 2008; Polte et al. 2008; Uthoff et al. 2003; Victor et al. 2010). Based on these and the findings in our study one may envision pre-conceptional, early post-conceptional passive immunization or other forms of administration of allergen-specific IgG (e.g., oral) as possible strategies for the prevention of allergy.
Experimental animal models of allergy are useful to study certain aspects of allergy (Helm and Burks 2004) but unfortunately do not completely resemble allergy in humans. For example, it is well established that mice recognize different epitopes on allergens compared to allergic patients. Furthermore, respiratory allergy in mice does not resemble human disease because generally high doses of allergens need to be administered to obtain airway inflammation which then involves mechanisms of inflammation distinct from those operative in humans (i.e., mainly T cell-mediated inflammation) (Epstein 2004). Yet our results demonstrate that passive immunization with allergen-specific IgG antibodies had a profound inhibitory effect on IgE-mediated effector cell degranulation, which is a major mechanism of allergy in allergic patients and on the induction of allergic sensitization. Since IgE epitopes on allergens recognized by mice and man are different (Epstein 2004) it will be necessary to isolate human allergen-specific IgG antibodies which block the binding of allergic patients IgE and to perform first clinical studies directly in allergic patients after careful evaluation of eventual harmful effects of therapeutic antibodies in toxicology studies rather then in laborious but incomplete allergy models in animals. In fact it has been shown that human antibodies with a suitable blocking activity even for polyclonal IgE responses can be identified (Flicker et al. 2002; Visco et al. 1996). Once these antibodies can be produced in recombinant form and suitable quality for safe clinical trials, it should be possible to evaluate whether passive immunization with allergen-specific IgG antibodies may be an effective strategy for the treatment of allergy as well as for the prevention of allergic sensitization.
Acknowledgements
This study was supported by grants F4605, F4607, F4612, P23318-B11 and P23350-B11 of the Austrian Science Fund (FWF), by grant 813003 of the Austrian Research Promotion Agency (FFG) and a research grant from Biomay, Vienna, Austria.
References
- Bernstein I.L., Michael J.G., Malkiel S., Sweet L.C., Brackett Immunoregulatory function of specific IgG. II. Clinical evaluation of combined active and passive immunotherapy. Int. Arch. Allergy Appl. Immunol. 1979;58:30–37. [PubMed] [Google Scholar]
- Bousquet J., Fontez A., Aznar R., Robinet-Levy M., Michel F.B. Combination of passive and active immunization in honeybee venom immunotherapy. J. Allergy Clin. Immunol. 1987;79:947–954. doi: 10.1016/0091-6749(87)90245-4. [DOI] [PubMed] [Google Scholar]
- Breiteneder H., Pettenburger K., Bito A., Valenta R., Kraft D., Rumpold H., Scheiner O., Breitenbach M. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–1938. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R.A., Barnard J.H., Hebald S., Stull A. Serological evidence of immunity with co-existing sensitization in a type of human allergy (hay fever) J. Exp. Med. 1935;62:733–750. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M.M. Do mouse models of allergic asthma mimic clinical disease? Int. Arch. Allergy Immunol. 2004;133:84–100. doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- Flicker S., Gadermaier E., Madritsch C., Valenta R. Passive immunization with allergen-specific antibodies. Curr. Top. Microbiol. Immunol. 2011;352:141–159. doi: 10.1007/82_2011_143. [DOI] [PubMed] [Google Scholar]
- Flicker S., Marth K., Kofler H., Valenta R. Placental transfer of allergen-specific IgG but not IgE from a specific immunotherapy-treated mother. J. Allergy Clin. Immunol. 2009;124 doi: 10.1016/j.jaci.2009.09.024. 1358–1360 e1351. [DOI] [PubMed] [Google Scholar]
- Flicker S., Steinberger P., Norderhaug L., Sperr W.R., Majlesi Y., Valent P., Kraft D., Valenta R. Conversion of grass pollen allergen-specific human IgE into a protective IgG(1) antibody. Eur. J. Immunol. 2002;32:2156–2162. doi: 10.1002/1521-4141(200208)32:8<2156::AID-IMMU2156>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Floistrup H., Swartz J., Bergstrom A., Alm J.S., Scheynius A., van Hage M., Waser M., Braun-Fahrlander C., Schram-Bijkerk D., Huber M., Zutavern A., von Mutius E., Ublagger E., Riedler J., Michaels K.B., Pershagen G., The Parsifal Study G. Allergic disease and sensitization in Steiner school children. J. Allergy Clin. Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Fusaro A.E., Brito C.A., Victor J.R., Rigato P.O., Goldoni A.L., Duarte A.J., Sato M.N. Maternal–fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology. 2007;122:107–115. doi: 10.1111/j.1365-2567.2007.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glovsky M.M., Ghekiere L., Rejzek E. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Ann. Allergy. 1991;67:21–24. [PubMed] [Google Scholar]
- Helm R.M., Burks A.W. Sensitization and allergic response and intervention therapy in animal models. J. AOAC Int. 2004;87:1441–1447. [PubMed] [Google Scholar]
- Jarrett E.E., Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49–58. [PMC free article] [PubMed] [Google Scholar]
- Jenmalm M.C., Bjorksten B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin. Exp. Allergy. 2000;30:34–40. doi: 10.1046/j.1365-2222.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- Laffer S., Valenta R., Vrtala S., Susani M., van Ree R., Kraft D., Scheiner O., Duchene M. Complementary DNA cloning of the major allergen Phl p I from timothy grass (Phleum pratense); recombinant Phl p I inhibits IgE binding to group I allergens from eight different grass species. J. Allergy Clin. Immunol. 1994;94:689–698. doi: 10.1016/0091-6749(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Larche M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Linhart B., Bigenzahn S., Hartl A., Lupinek C., Thalhamer J., Valenta R., Wekerle T. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J. Immunol. 2007;178:3924–3931. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless M. Immunological studies of pollinosis. I. The presence of two antibodies related to the same pollen antigen in the serum of treated hay fever patients. J. Immunol. 1940;38:25–50. [Google Scholar]
- Moerch U., Haahr Hansen M., Vest Hansen N.J., Rasmussen L.K., Oleksiewicz M.B., Frandsen T.P., Haurum J.S., Bregenholt S. Allergen-specific polyclonal antibodies reduce allergic disease in a mouse model of allergic asthma. Int. Arch. Allergy Immunol. 2006;140:261–269. doi: 10.1159/000093283. [DOI] [PubMed] [Google Scholar]
- Muller U.R., Morris T., Bischof M., Friedli H., Skarvil F. Combined active and passive immunotherapy in honeybee-sting allergy. J. Allergy Clin. Immunol. 1986;78:115–122. doi: 10.1016/0091-6749(86)90123-5. [DOI] [PubMed] [Google Scholar]
- Niederberger V., Laffer S., Froschl R., Kraft D., Rumpold H., Kapiotis S., Valenta R., Spitzauer S. IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Bet v 2) account for a high percentage of grass pollen-specific IgE. J. Allergy Clin. Immunol. 1998;101:258–264. doi: 10.1016/s0091-6749(98)70391-4. [DOI] [PubMed] [Google Scholar]
- Niederberger V., Pauli G., Gronlund H., Froschl R., Rumpold H., Kraft D., Valenta R., Spitzauer S. Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J. Allergy Clin. Immunol. 1998;102:579–591. doi: 10.1016/s0091-6749(98)70273-8. [DOI] [PubMed] [Google Scholar]
- Padavattan S., Flicker S., Schirmer T., Madritsch C., Randow S., Reese G., Vieths S., Lupinek C., Ebner C., Valenta R., Markovic-Housley Z. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J. Immunol. 2009;182:2141–2151. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- Pauli G., Larsen T.H., Rak S., Horak F., Pastorello E., Valenta R., Purohit A., Arvidsson M., Kavina A., Schroeder J.W., Mothes N., Spitzauer S., Montagut A., Galvain S., Melac M., Andre C., Poulsen L.K., Malling H.J. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Polte T., Hansen G. Maternal tolerance achieved during pregnancy is transferred to the offspring via breast milk and persistently protects the offspring from allergic asthma. Clin. Exp. Allergy. 2008;38:1950–1958. doi: 10.1111/j.1365-2222.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- Polte T., Hennig C., Hansen G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J. Allergy Clin. Immunol. 2008;122 doi: 10.1016/j.jaci.2008.09.014. 1022–1030 e1025. [DOI] [PubMed] [Google Scholar]
- Sehra S., Pynaert G., Tournoy K., Haegeman A., Matthys P., Tagawa Y., Pauwels R., Grooten J. Airway IgG counteracts specific and bystander allergen-triggered pulmonary inflammation by a mechanism dependent on Fc gamma R and IFN-gamma. J. Immunol. 2003;171:2080–2089. doi: 10.4049/jimmunol.171.4.2080. [DOI] [PubMed] [Google Scholar]
- Shamji M.H., Durham S.R. Mechanisms of immunotherapy to aeroallergens. Clin. Exp. Allergy. 2011;41:1235–1246. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- Strait R.T., Morris S.C., Finkelman F.D. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J. Clin. Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthoff H., Spenner A., Reckelkamm W., Ahrens B., Wolk G., Hackler R., Hardung F., Schaefer J., Scheffold A., Renz H., Herz U. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 2003;171:3485–3492. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- Victor J.R., Muniz B.P., Fusaro A.E., de Brito C.A., Taniguchi E.F., Duarte A.J., Sato M.N. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol. 2010;11:11. doi: 10.1186/1471-2172-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visco V., Dolecek C., Denepoux S., Le Mao J., Guret C., Rousset F., Guinnepain M.T., Kraft D., Valenta R., Weyer A., Banchereau J., Labecque S. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J. Immunol. 1996;157:956–962. [PubMed] [Google Scholar]
- Vrtala S., Sperr W.R., Reimitzer I., Van Ree R., Laffer S., Müller W.D., Valent P., Lechner K., Rumpold H., Kraft D., Scheiner O., Valenta R. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl p V allergen. J. Immunol. 1993;151:4773–4781. [PubMed] [Google Scholar]
- Westritschnig K., Horak F., Swoboda I., Balic N., Spitzauer S., Kundi M., Fiebig H., Suck R., Cromwell O., Valenta R. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur. J. Clin. Invest. 2008;38:260–267. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]