Abstract
Though Elizabethkingia meningosepticum typically causes meningitis in neonates, its occurrence in adult is rare, with sixteen cases described worldwide. We report a case of E. meningosepticum meningitis in an immunocompetent adult. Bacterial identification was made a day earlier than conventional method by using matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) Vitek mass spectrometry RUO (VMS), which resulted in successful treatment with rifampin, trimethoprim-sulfamethoxazole, levofloxacin and minocycline.
Keywords: chryseobacterium, Elizabethkingia meningosepticum, flavobacterium, meningitis, MALDI-TOF mass spectrometry, Vitek
Case report
A 68-year-old woman with known hypertension and osteoarthritis was found down in her residential complex and brought to the emergency department on 15 July 2011. On physical examination she was afebrile (36.1 °C), normotensive (107/43) and tachycardic (95 beats/min), with an oxygen saturation of 98% on room air. Swollen, erythematous lower extremities with areas of induration and scattered bullae were noted. Although initially alert and oriented, within 4 h she became difficult to arouse and was not following commands. Her neurological exam was non-focal, with pupils equally round and reactive to light, normal muscle tone and dull deep tendon reflexes. Initial laboratory findings were consistent with an anion-gap metabolic acidosis, acute renal failure, elevated liver enzymes and creatine phosphate kinase (Table 1). Head computed tomography imaging was unremarkable at the time of presentation. The patient was emergently intubated, and surgical exploration of her lower extremities was performed. No signs of necrotizing fasciitis were noted.
Table 1. Laboratory evaluation.
| Test | Result | Reference range |
|---|---|---|
| Chemistry | ||
| Sodium | 131 | 132–144 mEq/L |
| Potassium | 3.7 | 3.4–5.1 mEq/L |
| Chloride | 98 | 101–111 mEq/L |
| Bicarbonate | 17 | 22–32 mEq/L |
| Urea nitrogen | 62 | 8–22 mEq/L |
| Creatinine | 3.8 | 0.4–1.0 mg/dL |
| AST | 180 | 10–42 U/L |
| ALT | 70 | 14–54 U/L |
| Alkaline phosphatase | 73 | 38–141 U/L |
| Total bilirubin | 1.1 | 0.3–1.6 mg/dL |
| Creatine phosphate kinase | 4367 | 38–234 U/L |
| Hematology | ||
| WBC | 9.2 | 4.0×103–8.5×103 /μL |
| %Neutrophils | 81 | 25%–62% |
| %Bands | 18 | <13% |
| RBC | 4.1 | 3.9×106–5.2×106 /μL |
| Hemoglobin | 11.7 | 11.5–15.5 g/dL |
| Hematocrit | 35.1 | 35.0%–45.0% |
| Platelet count | 172 | 140×103–440×103 /μL |
| Cerebrospinal fluid | ||
| WBC | 0 | <11/μL |
| RBC | 28 | 0 μL |
| Protein | 67 | 15–45 mg/dL |
| Glucose | 53 | 40–70 mg/dL |
Abbreviations: AST, aspartate aminotransferase test; ALT, alanine aminotransferase; WBC, white blood cell; RBC, red blood cell.
On post-operative reevaluation, the patient exhibited left horizontal gaze palsy, a left dilated non-reactive pupil, extensor Babinski response bilaterally and decerebrate posturing. Repeat head computed tomography imaging 20 h after the initial scan showed interval development of hydrocephalus requiring placement of an external ventricular drain. Interestingly, the initial cerebrospinal fluid (CSF) sample analysis on external ventricular drain placement yielded zero white blood cells, mildly elevated protein levels and normal glucose levels. However, Gram-negative rods were noted on microscopy (data not shown). Although she had already received doses of vancomycin, piperacillin-tazobactam, ceftriaxone and ampicillin by the end of day 1, the patient developed septic shock.
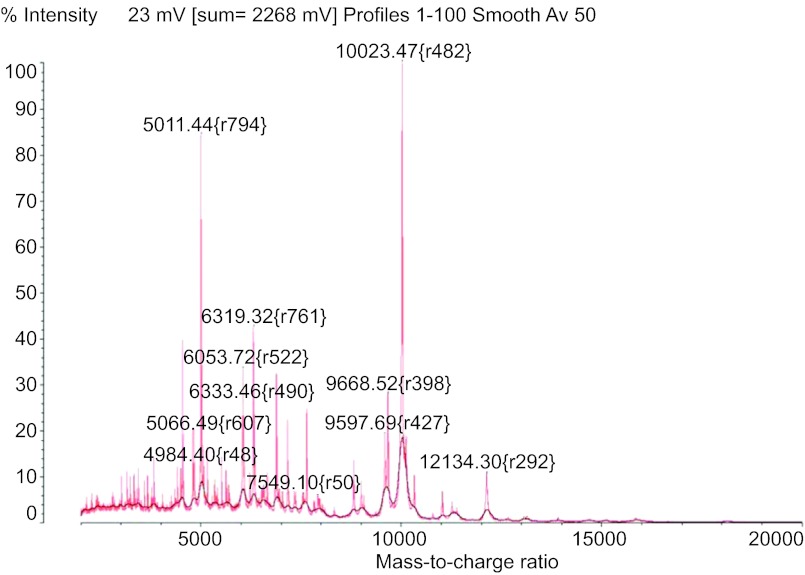
Within 24 h of admission, initial blood culture collected in emergency department, tissue and CSF cultures collected later were processed turned positive for the Gram-negative rod. Instead of waiting for another day for bacterial identification by using conventional culture method, the Gram-negative rod was identified as Elizabethkingia meningosepticum on the same day of positive culture by using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) Vitek mass spectrometry (VMS). The spectrum of VMS is shown in Figure 1. The patient's antimicrobial regimen was subsequently changed on the evening of hospital day 2, to levofloxacin 750 mg daily, trimethoprim-sulfamethoxazole 400 mg intravenous every 8 h, minocycline 100 mg every 12 h and rifampin 600 mg every 24 h. The regimen was derived from a review of the literature, 24 h prior to obtaining the conventional microbiological identification and sensitivities. The pathogen was susceptible to fluoroquinolones (minimal inhibitory concentration (MIC)≤1 µg/mL for ciprofloxacin and <2 µg/mL for levofloxacin) and trimethoprim-sulfamethoxazole (MIC≤2–38 µg/mL), but was resistant to aminoglycosides, imipenem and piperacillin-tazobactam by using MicroScan WalkAway (Siemens, West Sacramento, CA, USA) with LabPro software and Clinical and Laboratory Standards Institute guidelines.1 The patient's clinical status progressively improved, and within 72 h starting a tailored antibiotic regimen, her vital signs normalized.
Figure 1.
E. meningosepticum spectral signature from Vitek MS with SARAMIS RUO software.
Although this patient's initial neurological findings portended a poor prognosis, she exhibited early signs of neurological recovery with spontaneous movement of her extremities and head, eye tracking, as well as facial grimacing with withdrawal to pain. Magnatic resonance imaging of the brain performed on hospital day 6 revealed leptomeningeal enhancement compatible with a diagnosis of meningitis, but no other findings that could explain here neurological status. After a hospital course of 2 weeks, she was discharged to a long-term acute care hospital where she received 6 weeks of levofloxacin, minocycline and trimethoprim-sulfamethoxazole. By 8 weeks post-discharge, the patient had become alert and oriented and was actively participating in physical and occupational therapy, albeit with residual receptive aphasia.
Discussion
E. meningosepticum, previously known as Flavobacterium and reclassified as Chryseobacterium,2 was initially identified by Dr Elizabeth King as a causative agent of meningitis in neonates.3 E. meningosepticum infections, whether in infants or adults, are mostly nosocomial, with less than 15% acquired in the community.4,5 Case reports have described patients with endocarditis, cellulitis, necrotizing fasciitis, hepatitis, osteomyelitis and eye infections, in addition to the more common E. meningosepticum bacteremia and pneumonia.4 The majority of infections in neonates present as meningitis. However, E. meningosepticum meningitis in adults is rare. Only 16 cases have been described worldwide; 15 of these cases described patients who had underlying comorbidities or who had undergone surgical procedures (Table 2).
Table 2. Characteristics of E. meningosepticum meningitis reported cases in adults.
| References | Age, Sex | Underlying condition | Treatment | Outcome | Source |
|---|---|---|---|---|---|
| 15 | 17, Male | Thalassemia major, splenectomy | Vancomycin for 21 days | Cured | NR* |
| 16 | 88, Female | Diabetes mellitus, cellulitis | NR | Died | Community |
| 11 | 21, Female | Diabetes mellitus | Cefepime for 21 days | Cured | Community |
| 17 | 27, Female | Acute myelogenous leukemia | Rifampicin, piperacillin, ciprofloxacin for 23 days | Cured | Nosocomial |
| 18 | − | − | − | − | − |
| 19 | NR | Myelography | NR | NR | Nosocomial |
| 8 | 43, Male | NR | Piperacillin, cefoperazone, minocyclineerythromycin | Died | Community |
| 20 | 56, Female | Pituitary tumor s/p transphenoidal hypophysectomy | Rifampin, cefoperazone, chloramphenicol | Cured | Nosocomial |
| 21 | 60, Male | Squamous cell cancer s/p resection | Amikacin, erythromycin for 25d | Cured | Nosocomial |
| 22 | 26, Female | CKD, renal transplant, tuberculosis | Erythromycin | Died | Nosocomial |
| 23 | 66, Female | Acute myelogenous leukemia | Erythromycin | Died | Nosocomial |
| 24 | 43, Male | Squamous cell cancer s/p irradiation/resection | Ampicillin | Cured | Community |
| 25 | 19, Male | Aplastic anemia | Neomycin IV/intrathecal | Died | Nosocomial |
| 26 | 33, Male | Pulmonary tuberculosis, malnutrition | Chloramphenicol, erythromycin | Died | NR |
| 27 | NR | Postpartum | NR | NR | NR |
| 3 | NR | Polycythemia | NR | Cured | NR |
Abbreviations: CKD, chronic kidney disease; NR, not reported; − unavailable
E. meningosepticum is found ubiquitously in freshwater, saltwater and soil.6 However, the majority of E. meningosepticum infections are nosocomial in origin.4,5 As a biofilm-forming organism, it commonly colonizes sink drains and medical equipment such as ventilators, intravascular catheters and surgical tools, as well as solutions such as chlorhexidine.7 Hospital isolates are thought to be the underlying cause of sporadic nosocomial outbreaks. Positive screening cultures should, however, be interpreted cautiously, as only 60% have shown clinical correlation with an infectious process.5 Risk factors for E. meningosepticum infections relate to underlying immune dysfunction. In neonates, the main risk factor for E. meningosepticum infection is prematurity.5 Most adults with E. meningosepticum meningitis suffer from comorbidities such as hematologic malignancies, diabetes or have recently undergone surgical procedures (Table 2). In two retrospective case studies of E. meningosepticum bacteremia, the most common comorbidities were cancer and diabetes mellitus.4 Mortality rates in these patients are as high as 53%.4,5 Although one case involved a 46-year-old man with no known underlying systemic disorder who succumbed to meningitis,8 no immunologic evaluation was performed prior to his death. Our patient had regular follow-up visits with her primary care physician. Screening colonoscopy and mammogram were completed the year prior to presentation and were unremarkable. She had never been on corticosteroids. HIV antibody and a viral hepatitis panel were negative. Her hemoglobin A1C was 6.0%. Serum immunoglobulin and complement levels were within normal limits and anti-nuclear antibody screen was non-reactive. Thus, E. meningosepticum meningitis can occur in an immunocompetent host.
E. meningosepticum's unusual antimicrobial sensitivity is due to its production of two different metallo-β-lactamases, conferring the ability to degrade all β-lactam antibiotics.9 Antibiotic susceptibility profiles vary across the reported literature4,5 and thus, there is no consensus on appropriate therapy. The largest study examining 99 isolates and their susceptibilities to 19 antimicrobial agents suggested that E. meningosepticum is most sensitive to trimethoprim-sulfamethoxazole (91%), followed levofloxacin and moxifloxacin (81%–87%), doxycycline and piperacillin-tazopactam (78%).4 The SENTRY report examining 24 isolates noted 87% susceptibility to rifampin.10 Aminoglycosides and vancomycin have shown poor activity against E. meningosepticum.4
The CSF findings in this case illustrate potential diagnostic challenges. Three out of 10 cases reporting CSF findings of adult patients with E. meningosepticum meningitis showed a paucity of CSF inflammation as low as 6 white blood cells (WBCs)/µL.11 In this patient's case, there were 0 WBCs/µl in the initial CSF obtained during extra-ventricular drain insertion, despite positive Gram stain and culture. These findings show that meningitis caused by this organism cannot be ruled out with a very low or even normal CSF WBC count.
Rapid bacteriologic identification is essential as E. meningosepticum is typically resistant to the common antimicrobials used to empirically treat Gram-negative rod infections. MALDI-TOF Mass Spectrometry (MS) technology provided the diagnosis within 24 h of admission. Its utility in protein profiling has emerged as a powerful tool for the rapid identification of bacteria and yeast isolates.12 MALDI-TOF MS can be performed very quickly, requiring a mean of a few minutes per sample to identify an isolate, and with high accuracy.13 In this case, MALDI-TOF VMS aided management by providing an early identification of an unusual and inherently resistant organism, allowing adjustment of the antibiotic regimen (Table 3). The Vitek MS RUO System with SARAMIS database by bioMérieux (Durham, NC, USA) is a research use only MALDI-TOF MS system for rapid detection of bacterial and yeast isolates. The MALDI-TOF VMS analysis was performed by using the Shimadzu instrument and the SARAMIS database originally developed by AnagnosTec GmbH and later acquired by bioMérieux.14 With this technique, colonies from the bacterial culture plate were placed on disposable VMS target plates (cat. no. 220-99999-FM1; Shimadzu Biotech, Columbia, MD, USA) by using disposable loop, overlaid with 1 µl α-cyano-4-hydroxycinnamic acid matrix (bioMérieux cat. no. 411071), and then air dried before being processed by the spectrometer. The result is a spectral fingerprint which is unique to each species, as the mass peaks reflect ribosomal and other constitutive proteins (Figure 1). The spectral signature is cross-referenced in a database to identify the organism according to its genus or species. The percent of confidence identification ranged from 76% to 89.9%. Within 24 h, the result was confirmed by conventional method using MicroScan (Siemens, West Sacramento, CA, USA) Gram-negative identification panel with 99.9% probability of E. meningosepticum and later by Vitek2 Gram-negative card (bioMerieux). In general, SARAMIS confidence level of ≥75% is acceptable for identification. For confirmation of bacterial identification by conventional method such as MicroScan, the isolate was sent to another laboratory (Dr Xiang-Yang Han of MD Anderson in Houston, TX, USA) for 16S ribosomal RNA sequencing. It was identified as Elizabethkingia meningoseptica with a score of 1056 bits (1170) and identities 587/588 (99%). MALDI-TOF MS was performed for all isolates; blood, CSF, tissue, as well as sputum samples, identifying the same bacterium with identical spectrum, as well as antimicrobial susceptibility profile.
Table 3. Timeline of bacterial identification.
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Blood was collected | Blood culture was positive, and subculture to plate | Positive growth, identified by MALDI-TOF MS | Identification by conventional method | Antimicrobial susceptibility testing by conventional method |
| CSF was collected | Positive growth, identified by MALDI-TOF MS | Identification by conventional method | Antimicrobial susceptibility testing by conventional method |
Abbreviations: CSF, cerebrospinal fluid.
Conclusion
E. meningosepticum is a virulent pathogen, not only in the immunocompromised host, but also in immunocompetent patients. Clinical and laboratory manifestations of E. meningosepticum infections are not pathognomonic; thus, early microbiological diagnosis using emerging automated technology such as MALDI-TOF MS is essential in selecting appropriate therapy. As the available data suggest 81% to 91% of isolates are sensitive to trimethoprim-sulfamethoxazole, levofloxacin and rifampin, early identification could predict the antimicrobial susceptibility pattern and help clinicians to choose the right antibiotics.
References
- Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement M100-S21. Wayne; Clinical and Laboratory Standards Institute, 2011; [Google Scholar]
- Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55 Pt 3:1287–1293. doi: 10.1099/ijs.0.63541-0. [DOI] [PubMed] [Google Scholar]
- King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- Hsu MS, Liao CH, Huang YT, et al. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical center in Taiwan, 1999–2006. Eur J Clin Microbiol Infect Dis. 2011;30:1271–1278. doi: 10.1007/s10096-011-1223-0. [DOI] [PubMed] [Google Scholar]
- Bloch KC, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine. 1997;76:30–41. doi: 10.1097/00005792-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Bernardet JF, Hugo C, Bruun B.The genera Chryseobacterium and ElizabethkingiaIn: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (ed.)The Prokaryotes: a Handbook on the Biology of Bacteria. Proteobacteria: Delta and Epsilon Subclasses. Deeply Rooting Bacteria. Vol 7. 3rd ed New York; Springer ; 2006638–676. [Google Scholar]
- Coyle-Gilchrist MM, Crewe P, Roberts G. Flavobacterium meningosepticum in the hospital environment. J Clin Pathol. 1976;29:824–826. doi: 10.1136/jcp.29.9.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihara T, Yokota T, Watabiki S, Ueki M, Miyake S, Tsukagoshi H. Flavobacterium meningosepticum meningitis in an adult. Am J Med. 1988;85:738–739. doi: 10.1016/s0002-9343(88)80257-2. [DOI] [PubMed] [Google Scholar]
- Vessillier S, Docquier JD, Rival S, et al. Overproduction and biochemical characterization of the Chryseobacterium meningosepticum BlaB metallo-beta-lactamase. Antimicrob Agents Chemother. 2002;46:1921–1927. doi: 10.1128/AAC.46.6.1921-1927.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JT, Sader HS, Walsh TR, Jones RN. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997–2001) J Clin Microbiol. 2004;42:445–448. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CH, Huang CR, Tsai NW, et al. An adult case of Chryseobacterium meningosepticum meningitis. Jpn J Infect Dis. 2004;57:214–215. [PubMed] [Google Scholar]
- Carbonnelle E, Mesquita C, Bille E, et al. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem. 2011;44:104–109. doi: 10.1016/j.clinbiochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Seng P, Drancourt M, Gouriet F, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- Emonet S, Shah HN, Cherkaoui A, Schrenzel J. Application and use of various mass spectrometry methods in clinical microbiology. Clin Microbiol Infect. 2010;16:1604–1613. doi: 10.1111/j.1469-0691.2010.03368.x. [DOI] [PubMed] [Google Scholar]
- Ozkalay N, Anil M, Agus N, Helvaci M, Sirti S. Community-acquired meningitis and sepsis caused by Chryseobacterium meningosepticum in a patient diagnosed with thalassemia major. J Clin Microbiol. 2006;44:3037–3039. doi: 10.1128/JCM.00588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmaja P, Verghese S, Bhirmanandham CV, Thirugnanasambandham AjithS, Ramesh S. Chryseobacterium meningosepticum—an uncommon pathogen causing adult bacterial meningitis. Indian J Pathol Microbiol. 2006;49:293–295. [PubMed] [Google Scholar]
- Krebs S, Blanche P, Bouscary D, et al. Flavobacterium meningosepticum meningitis in an adult with acute leukaemia. Postgrad Med J. 1996;72:187–188. doi: 10.1136/pgmj.72.845.187-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascunana A, Marin P, Pastorin J, Giron JA.[Meningitis caused by Flavobacterium meningosepticum in an adult] Enferm Infecc Microbiol Clin 199614394–395.Spanish. [PubMed] [Google Scholar]
- Bo SH, Nestvold K, Sortland O.[Meningitis after myelography] Tidsskr Nor Laegeforen 19951152646–2647.Spanish [PubMed] [Google Scholar]
- Chan KH, Chau PY, Wang RY, Huang CY. Meningitis caused by Flavobacterium meningosepticum after transsphenoidal hypophysectomy with recovery. Surg Neurol. 1983;20:294–296. doi: 10.1016/0090-3019(83)90082-4. [DOI] [PubMed] [Google Scholar]
- Harrington SP, Perlino CA. Flavobacterium meningosepticum sepsis: disease due to bacteria with unusual antibiotic susceptibility. South Med J. 1981;74:764–766. [PubMed] [Google Scholar]
- Mani RM, Kuruvila KC, Batliwala PM, et al. Flavobacterium meningosepticum as an opportunist. J Clin Pathol. 1978;31:220–222. doi: 10.1136/jcp.31.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios I, Klimek JJ, Maderazo E, Quintiliani R. Flavobacterium meningosepticum meningitis: report of selected aspects. Antimicrob Agents Chemother. 1978;14:444–447. doi: 10.1128/aac.14.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagely DH, Jr, Alexander JC, Jr, Gill VJ, Dolin R, Ketcham AS. Late flavobacterium species meningitis after craniofacial exenteration. Arch Intern Med. 1976;136:229–231. doi: 10.1001/archinte.136.2.229. [DOI] [PubMed] [Google Scholar]
- Lapage SP, Owen RJ. Flavobacterium meningosepticum from cases of meningitis in Botswana and England. J Clin Pathol. 1973;26:747–749. doi: 10.1136/jcp.26.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madruga M, Zanon U, Pereira GM, Galvao AC. Meningitis caused by Flavobacterium meningosepticum. The first epidemic outbreak of meningitis in the newborn in South America. J Infect Dis. 1970;121:328–330. doi: 10.1093/infdis/121.3.328. [DOI] [PubMed] [Google Scholar]
- Shibata O, Mitsuma T. A case of meningitis due to Flavobacterium meningosepticum. Jpn J Med. 1966;55:53. [Google Scholar]