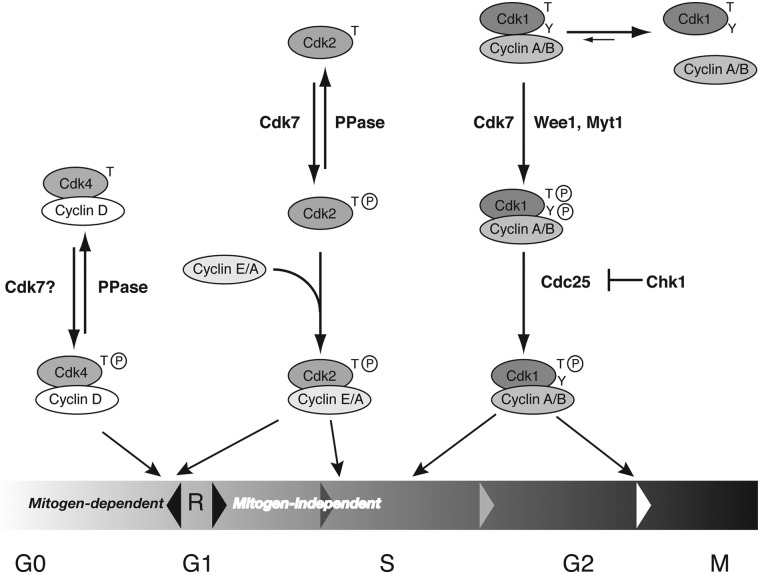
Figure 1.
Distinct activation pathways for different CDKs. In this model of the mammalian cell cycle, only a single CAK, Cdk7, is required, because of its ability to support distinct activation pathways for effector CDKs needed at different times. Prior to passage of the R point in G1, cell-cycle progression is reversible and dependent on continuous mitogenic signaling. Both Cdk4/cyclin D and Cdk2/cyclin E are required for R-point passage, and both might follow predominant activation pathways that are “reversible” by a cellular phosphatase (PPase). Cdk7 can overcome this opposition to activate Cdk2 by virtue of a faster rate of T-loop phosphorylation on monomeric substrates; I hypothesize a similar rate enhancement to allow activation of Cdk4/cyclin D complexes (see text; Cdk6 is omitted for clarity). Cdk2 also functions downstream of the R point, when progression through the cell cycle becomes mitogen-independent and irreversible; it has priority over Cdk1 in binding cyclin A because of its phosphorylation-first activation pathway. In the case of Cdk1, cyclin-binding and T-loop phosphorylation are mutually dependent and must occur in concert, coupled with inhibitory phosphorylations by kinases such as Wee1 and Myt1. This makes Cdk1 activation at the G2/M boundary dependent on removal of inhibitory Tyr phosphorylation by Cdc25, which is held in check by Chk1.