Figure 2.
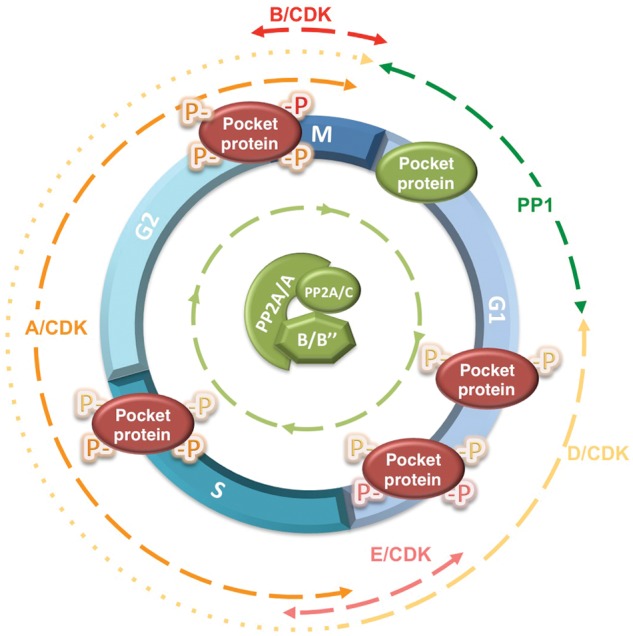
The phosphorylation state of pocket proteins is regulated by an equilibrium between inducible cyclin/CDK complexes and 2 major types of Ser/Thr phosphatases. The active, hypophosphorylated form of pocket proteins is shown in green, and the inactive, hyperphosphorylated pocket protein is shown in red. Although PP1 has been implicated in dephosphorylation of pRB from late mitosis to G1, when CDK activity is low, PP2A appears to restrict pocket protein phosphorylation throughout the cell cycle and in quiescent cells (not shown in this figure). Two heterotrimeric complexes have been directly implicated in dephosphorylation of pocket proteins, PP2A/B55α and PP2A/PR70. PP2A/B55α was shown to preferentially target p107 and p130, but our unpublished observations indicate that pRB is also a target. PP2A/PR70 has been implicated in pRB dephosphorylation upon oxidative stress. However, its contribution to the phosphorylation state of pocket proteins in the absence of inducible signals has not been investigated. The approximate portion of the cell cycle in which each participating CDK and Ser/Thr phosphatase is active toward pocket proteins is indicated by dashed lines. Of note, the expression of D-type cyclins during the cell cycle, which is stimulated by mitogens, varies in different cell types. In some cell types, D-type cyclins are only expressed from mid-G1 through the G1/S transition (this is denoted by dashes changing to dots).
