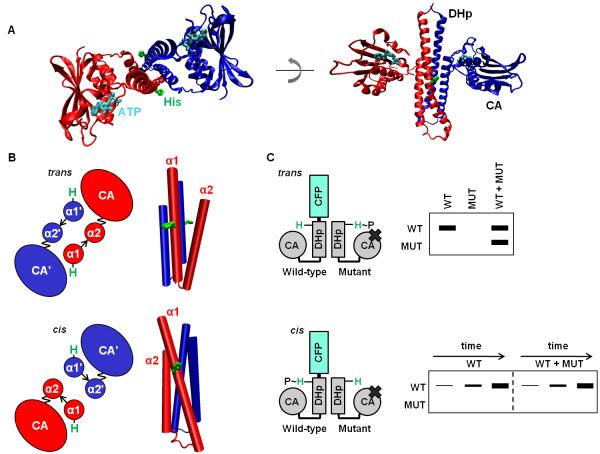
Figure 1. Histidine kinases autophosphorylate in cis or in trans.
(A) The cytoplasmic region of a histidine kinase (PDB ID 2C2A) consists of conserved DHp and CA domains. The histidine site of phosphorylation on the DHp domain, and the ATP analog bound by the CA domain, are shown in stick form in green and cyan, respectively. The kinase shown autophosphorylates in cis. (B) Cartoon (left) looking down the four-helix bundle (right) of the DHp-domain dimer. The α1 and α2 helices in the DHp domain are labeled, with the prime symbol (’) symbol denoting the opposite chain (top: PDB ID 3ZRX, bottom: PDB ID 2C2A). The loop at the base of the DHp domain is depicted by an arrow, and the linker between the DHp and CA domains is depicted as a wavy line to reflect the mobility of the CA domain. Depending on loop handedness in the DHp domain, the CA domain is either closer to the histidine on the same chain (cis) or the histidine on the opposite chain (trans). (C) Schematic of the assay to test in cis vs. in trans autophosphorylation. A wild-type (WT) histidine kinase homodimer is mixed with excess mutant (MUT) histidine-kinase homodimer unable to bind ATP. Autophosphorylation within the heterodimer is initiated by the addition of radiolabeled ATP, and the chains in the dimer are then separated by SDS-PAGE. In the heterodimer, either the WT or MUT chain is labeled, depending on whether the kinase autophosphorylates in cis or in trans, respectively. In addition, WT homodimer, also present in the WT+MUT mixture, undergoes autophosphorylation. To confirm autophosphorylation in cis, the kinetics of the WT and WT plus excess MUT reactions are compared.