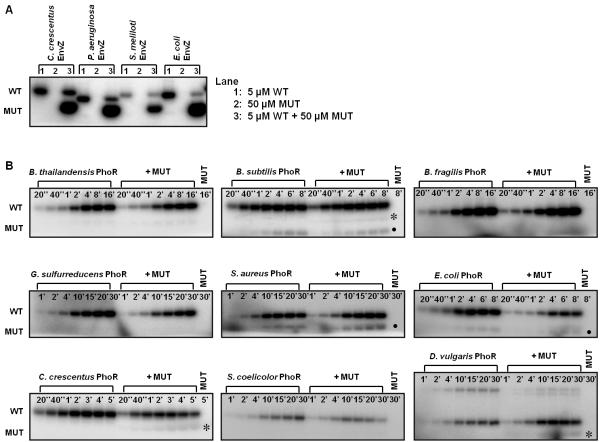
Figure 3. Autophosphorylation mechanism within EnvZ and PhoR orthologs is conserved.
(A) Characterization of autophosphorylation mechanism for EnvZ orthologs. In each ortholog series, the first gel lane contained 5 μM wild-type (WT) kinase, the second lane contained 50 μM mutant (MUT) kinase, and the third lane contained a mixture of the two kinases. The mixture was incubated for four hours at 30 °C before initiating autophosphorylation. (B) Characterization of autophosphorylation mechanism for PhoR orthologs. The gel for each ortholog included two autophosphorylation time courses: the first for 5 μM wild-type kinase alone and the second for 5 μM wild-type kinase plus 50 μM mutant kinase. The final lane contained 50 μM mutant kinase. The autophosphorylation time is marked in each lane. Asterisks indicate possible weak in trans autophosphorylation, and solid circles mark a breakdown product found in both the WT and WT + MUT reactions. The slower mobility band seen with D. vulgaris PhoR is a PhoR dimer. Gel images were quantified and are plotted in Supplementary Fig. 2.