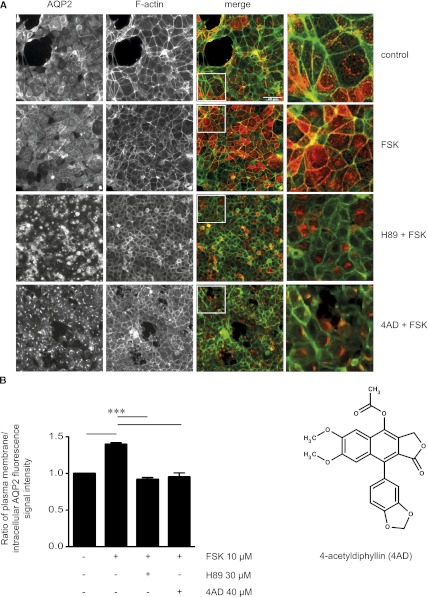
Figure 1.
Identification of 4AD as an inhibitor of the forskolin (FSK)-induced AQP2 redistribution in MCD4 cells by cell-based high-throughput screening. (A) MCD4 cells were seeded into 384-well plates, left untreated, treated with forskolin (10 µM, 20 minutes), or incubated with each of the 17,700 small molecules from the ChemBioNet small-molecule library (40 µM) for 100 minutes before forskolin (10 µM) was added for an additional 20 minutes. As a control, the forskolin-induced redistribution of AQP2 was inhibited with H89 (30 µM, 2 hours), a known blocker of the AQP2 translocation. The cells were fixed with paraformaldehyde, and AQP2 was detected by immunofluorescence microscopy using specific antibodies (H27) and Cy5-coupled antirabbit secondary antibodies (red).51 F-actin was visualized using TRITC-conjugated phalloidin (green). Fluorescence signals were detected using an automated screening microscope (10× magnification). The magnified views are from the indicated squares in the merge. Shown are representative images from one of three independent experiments. (B) The chemical structure represents 4AD. The intensities of intracellular and plasma membrane immunofluorescence signals arising from AQP2 were determined and related to nuclear signal intensities (mean ± SEM; three independent experiments). The ratios of plasma membrane to intracellular fluorescence signal intensities were calculated. According to the ratios determined for cells treated with forskolin alone (1.4) and forskolin in combination with H89 (0.9), ratios <1.2 indicate a predominant intracellular localization. Values significantly different from forskolin-treated cells are indicated (***P<0.001).