Table 1.
Small molecule inhibitors of the cAMP-induced redistribution of AQP2.
| No. | ID | Structure | Formula | Ratio | Plate | Well | Molecular Weight | Partition Coefficient | H-Acc | H-Don | IUPAC Nomenclature |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4-acetydiphillin (4AD) 216407 | 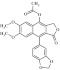 |
C23H18O8 | 0.88 | 115 | N9 | 422.4 | 3.08 | 6 | 0 | 9-benzo[d][1,3] dioxol- 5-yl)-6,7-dimethoxy-1-oxo-1,3-dihydronaphtho[2,3-c]furan-4-yl acetate |
| 2 | 212678 | 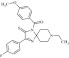 |
C23H24FN3O2S | 0.84 | 105 | K1 | 425.5 | 4.99 | 4 | 0 | (8-ethyl-3-(4-fluorophenyl)-2-thioxo-1,4,8-triazaspiro[4.5]dec-3-en-1-yl)(4-methoxyphenyl)methanone |
| 3 | 212634 | 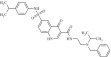 |
C31H36N4O4S | 0.88 | 104 | D14 | 560.7 | 5.2 | 6 | 3 | N-{2[benzyl (isopropyl)amino] ethyl}-6-[(4isopropylphenyl)sulfamoyl]-4-oxo-1H-quinoline-3-carboxamide |
| 4 | 202336 | 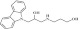 |
C18H22N2O2 | 1 | 75 | P11 | 298.4 | 1.69 | 3 | 3 | 3-((3-(9H-carbazol-9-yl) 2 hydroxypropyl)amino)propan-1-ol |
| 5 | 209614 triazolpropenon | 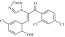 |
C17H10Cl3N3O2 | 1.06 | 96 | K6 | 394.6 | 4.58 | 4 | 1 | (Z)-3-(3-chloro-6-hydroxycyclohexa-2,4-dienyl)-1-(2,4-dichlorophenyl)-2-(11H,2,4-triazol-1-yl)prop-2-en-1-one |
| 6 | 214590 | 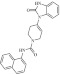 |
C23H20N4O2 | 0.69 | 110 | K18 | 384.4 | 3.24 | 2 | 2 | N-(naphthalen-1-yl)-4-(2-oxo-3H-1,3-benzodiazol-1-yl)-3,6-dihydro-2H-pyridine-1-carboxamide |
| 7 | 205653 | 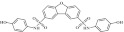 |
C24H18N2O7S2 | 0.78 | 85 | I5 | 510.5 | 3.52 | 6 | 4 | N,N′-bis(4-hydroxyphenyl)dibenzo[b,d]furan-2,8-disulfonamide |
| 8 | 216086 | 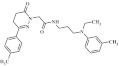 |
C25H32N4O2 | 0.79 | 114 | L17 | 420.5 | 3.47 | 4 | 1 | N-{3-[ethyl(3-methylphenyl)amino]propyl}-2-[3-(4-methylphenyl)-6-oxo-4,5-dihydropyridazin-1-yl]acetamide |
| 9 | 214645 | 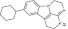 |
C19H23N3 | 0.8 | 110 | J9 | 293.4 | 3.62 | 2 | 1 | 9-cyclohexyl-2,3,5,6-tetrahydro-1H-3,4,6a-triazafluoranthene |
| 10 | 203240 | 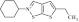 |
C13H18NS2 | 0.89 | 78 | O17 | 252.4 | 1.6 | 0 | 0 | 2-cyclohexyl-5-ethyl-2,3-dihydrothieno[3,2-d]isothiazole |
| 11 | 215223 | 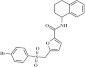 |
C22H20BrNO4S | 0.9 | 112 | M21 | 474.4 | 4.23 | 3 | 1 | 5-[(4-bromobenzenesulfonyl)methyl]-N-(1,2,3,4-tetrahydronaphthalen-1-yl)furan-2-carboxamide |
| 12 | 203313 | 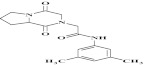 |
C17H21N3O3 | 1 | 78 | A16 | 315.4 | 0.79 | 3 | 1 | N-(3,5-dimethylphenyl)-2-(1,4 dioxohexahydropyrrolo[1,2-a] pyrazin-2(1H)-yl) acetamide |
| 13 | 201213 | 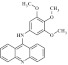 |
C22H20N2O3 | 1.02 | 72 | I18 | 360.4 | 4.47 | 5 | 1 | N-(3,4,5-trimethoxyphenyl)acridin-9-amine |
| 14 | 208677 | 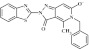 |
C21H15N4O2S | 1.07 | 93 | J13 | 387.4 | 1.13 | 5 | 0 | 2-(1,3-benzothiazol-2-yl)-5-benzyl-4-methyl-3-oxo-3,5-dihydro-2H-pyrazolo[4,3-c]pyridin-6-olate |
| 15 | 202329 | 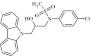 |
C22H21ClN2O3S | 1.12 | 75 | B11 | 428.9 | 3.69 | 3 | 1 | N-(3-(9H-carbazol-9-yl)-2-hydroxypropyl)-N-(4-chlorophe nyl)methane-sulfonamide |
| 16 | 204332 | 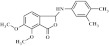 |
C18H19NO4 | 1.16 | 81 | G6 | 313.3 | 3.84 | 4 | 1 | 3-[(3,4dimethyl-phenyl)amino]-6,7-dimethoxy-3H-2-benzofuran-1-one |
| 17 | 209044 | 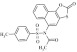 |
C20H15NO5S2 | 1.18 | 94 | H17 | 413.5 | 4.34 | 5 | 0 | N-(2-oxonaphtho[2,1-d][1,3]oxathiol-5-yl)-N-tosylacetamide |
Primary screening of 17,700 small molecules identified 83 inhibitors of the cAMP-induced redistribution of AQP2 in MCD4 cells. Secondary screening revealed that 17 chemically highly diverse hits inhibited the AQP2 redistribution in a concentration-dependent manner in MCD4 cells (compounds 1–17). Compounds 1–5 also inhibited the redistribution in IMCD cells. Compounds are ranked according to increasing ratios of plasma membrane/intracellular AQP2 fluorescence signal intensities. Compounds with ratios ≤ 1.2 were defined as inhibitory (a predominant intracellular localization of AQP2). Compound ID numbers (ID), compound library positions (plate, well), molecular weight, numbers of proton donor (H-Don.) and acceptor (H-Acc.) moieties are given for each substance. Compound names are according to the International Union of Pure and Applied Chemistry nomenclature. IUPAC, International Union of Pure and Applied Chemistry.
