Abstract
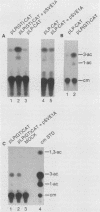
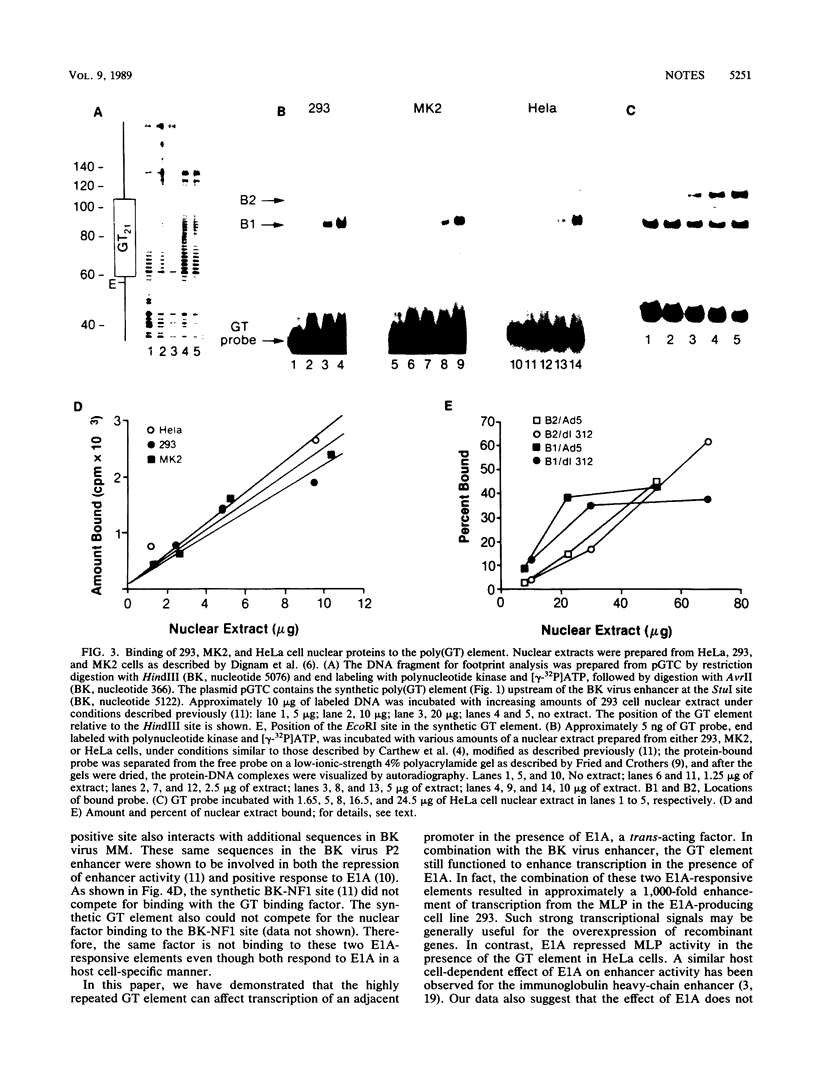
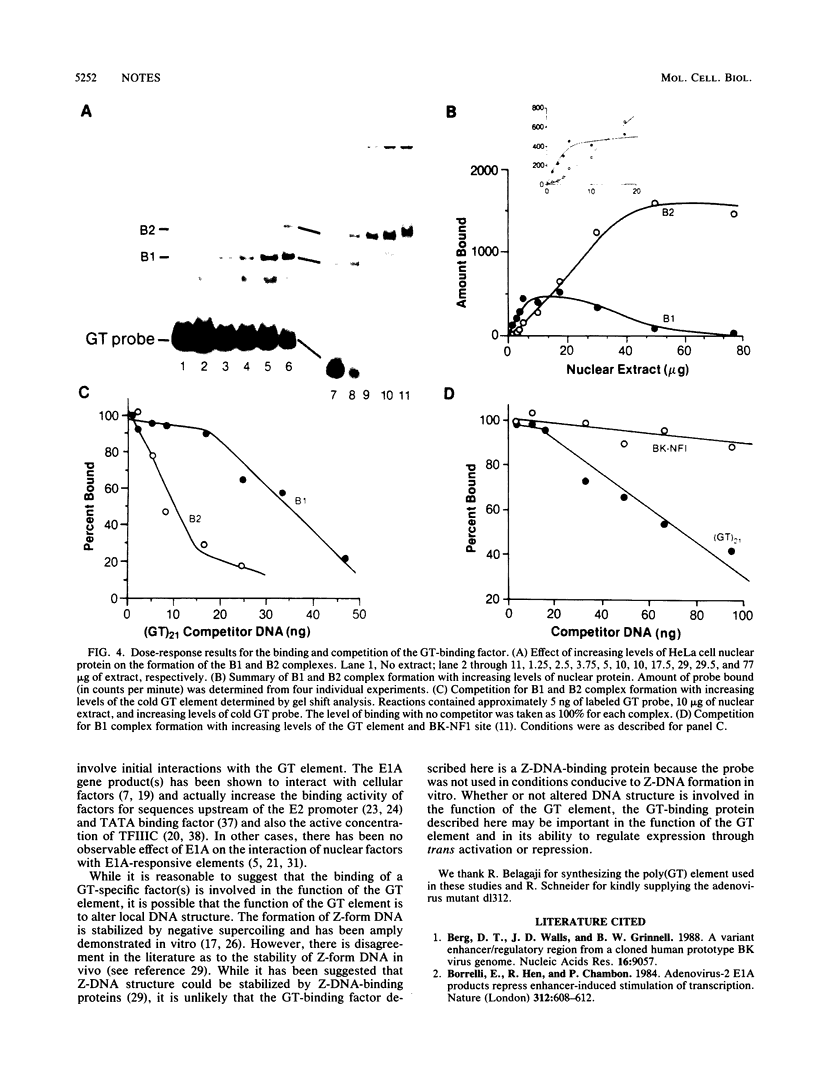
The alternating sequence poly(dG-dT).poly(dA-dC) is a highly repeated sequence in the eucaryotic genome. We have examined the effect of trans-acting early viral proteins on the ability of the GT element to stimulate transcription of the adenovirus major late promoter (MLP). We find that the GT element alone does not activate expression from the MLP in either the presence or absence of another enhancer element. However, in the presence of the E1A gene products of either adenovirus type 5 or 2, the GT element activated expression from the MLP. The stimulatory activity of the GT element in the presence of E1A had the properties of an enhancer element, and the trans-activating effect on the GT element was additive in conjunction with the E1A-responsive BK virus enhancer. We also have demonstrated that a specific nuclear factor(s) binds to the GT element. However, the E1A protein(s) do not affect the initial factor interaction(s) with the GT element. Overall, our data demonstrate that trans modulation of promoter activity can be mediated through the GT element.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. T., Walls J. D., Grinnell B. W. A variant enhancer/regulatory region from a cloned human prototype BK virus genome. Nucleic Acids Res. 1988 Sep 26;16(18):9057–9057. doi: 10.1093/nar/16.18.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Wasylyk C., Wasylyk B., Chambon P. The immunoglobulin heavy chain enhancer is stimulated by the adenovirus type 2 E1A products in mouse fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(9):2846–2849. doi: 10.1073/pnas.83.9.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux B., Albrecht G., Kedinger C. Identical genomic footprints of the adenovirus EIIa promoter are detected before and after EIa induction. Mol Cell Biol. 1987 Dec;7(12):4560–4563. doi: 10.1128/mcb.7.12.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Yee S. P., Ferguson B., Rosenberg M., Branton P. E. Binding of cellular polypeptides to human adenovirus type 5 E1A proteins produced in Escherichia coli. Virology. 1987 Sep;160(1):292–296. doi: 10.1016/0042-6822(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Berg D. T., Walls J. D. Negative regulation of the human polyomavirus BK enhancer involves cell-specific interaction with a nuclear repressor. Mol Cell Biol. 1988 Aug;8(8):3448–3457. doi: 10.1128/mcb.8.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Berg D. T., Walls J. Activation of the adenovirus and BK virus late promoters: effects of the BK virus enhancer and trans-acting viral early proteins. Mol Cell Biol. 1986 Nov;6(11):3596–3605. doi: 10.1128/mcb.6.11.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Huang S. Y., Garrard W. T. Chromatin structure of the potential Z-forming sequence (dT-dG)n X (dC-dA)n. Evidence for an "alternating-B" conformation. J Mol Biol. 1985 May 25;183(2):251–265. doi: 10.1016/0022-2836(85)90218-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Jalinot P., Kédinger C. Negative regulatory sequences in the EIa-inducible enhancer of the adenovirus-2 early EIIa promoter. Nucleic Acids Res. 1986 Mar 25;14(6):2651–2669. doi: 10.1093/nar/14.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. E1A transcription induction: enhanced binding of a factor to upstream promoter sequences. Science. 1986 Feb 14;231(4739):719–722. doi: 10.1126/science.2935935. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Markowitz R. B., Dynan W. S. Binding of cellular proteins to the regulatory region of BK virus DNA. J Virol. 1988 Sep;62(9):3388–3398. doi: 10.1128/jvi.62.9.3388-3398.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Campos A., Ellison M. J., Pérez-Grau L., Azorin F. DNA conformation and chromatin organization of a d(CA/GT)30 sequence cloned in SV40 minichromosomes. EMBO J. 1986 Jul;5(7):1727–1734. doi: 10.1002/j.1460-2075.1986.tb04417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Costanzo F., Ciliberto G. Inhibition of eukaryotic tRNA transcription by potential Z-DNA sequences. EMBO J. 1984 Jul;3(7):1553–1559. doi: 10.1002/j.1460-2075.1984.tb02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Recombination between poly[d(GT).d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985 Jun;5(6):1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Manley J. L. Structure and function of the S1 nuclease-sensitive site in the adenovirus late promoter. Cell. 1986 Jun 6;45(5):743–751. doi: 10.1016/0092-8674(86)90788-9. [DOI] [PubMed] [Google Scholar]