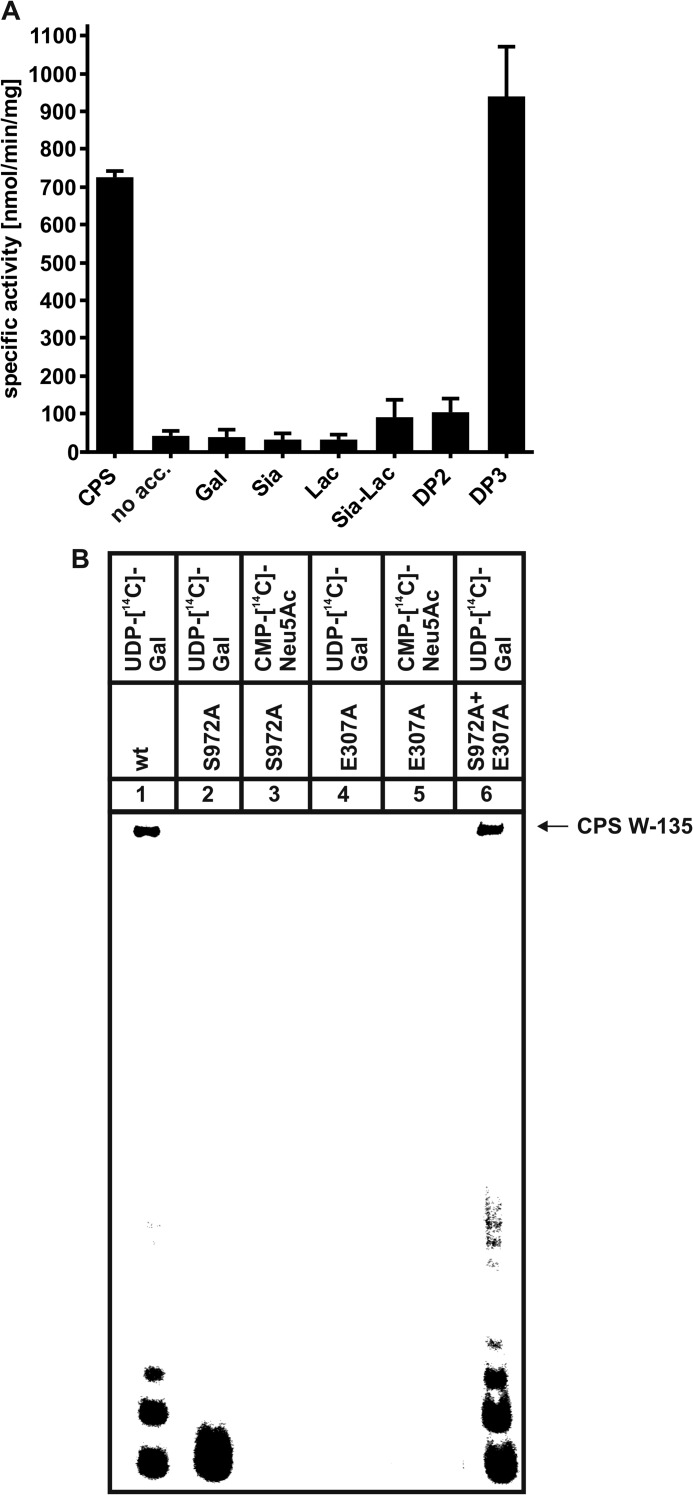
FIGURE 5.
A, the trimer of α(2,8)-linked sialic acid residues (DP3) is efficiently elongated by SiaDW-135. As defined W-135 CPS oligomers were not readily available, the compounds as listed were analyzed for their capacity to prime the SiaDW-135 reaction in the radioactive incorporation assay. In this experiment DP3 was found to be efficiently elongated by SiaDW-135, whereas DP2 and 2,3-sialyllactose (Sia-Lac) were only weak acceptors. The monosaccharides Gal and Sia as well as the disaccharide lactose (Lac) were not used by the enzyme. Specific activities were calculated from three independent experiments. B, asking if DP3 is an acceptor for both glycosyltransferase domains present in SiaDW-135, the transfer reactions catalyzed by the single mutants E307A and S972A were analyzed in comparison to the wild-type enzyme. Both nucleotide sugars were present in these reactions, with only one radioactively labeled as indicated. Long polymer chains were synthesized in reaction mixtures with wild-type SiaDW-135 (lane 1) and both single mutants (lane 6). In contrast, a single radioactive spot, representing the transfer of one [14C]Gal onto DP3 (lane 2) by S972A (comprises an active galactosyltransferase domain) was observed, whereas all other reactions (lanes 3-5) remained negative. These data clearly demonstrate that DP3 is selectively recognized by the galactosyltransferase domain and that formation of long CPS requires the iterative activity between galactosyl- and sialyltransferase domain.