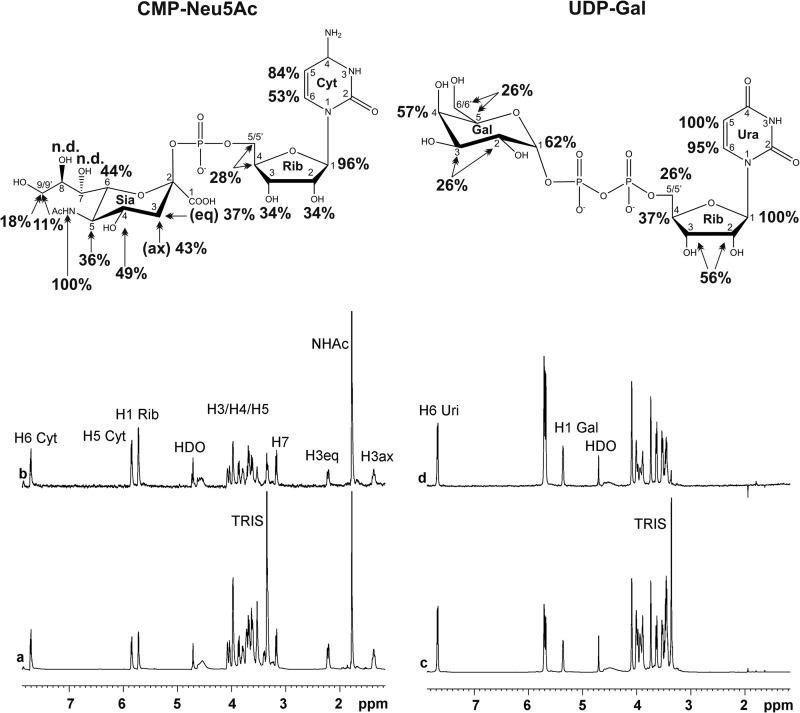
FIGURE 7.
STD NMR spectra of SiaDW-135 in complex with the nucleotide sugar donor substrates. To determine epitopes in the donor sugars CMP-Neu5Ac (a and b) and UDP-Gal (c and d) that make contact to the protein, an STD NMR study was performed. In line with previous data this experiment demonstrated the importance of H1 Rib for nucleotide sugar binding. This position together with H5 in the nucleotide base received the highest saturation in both UDP-Gal and CMP-Neu5Ac. In accordance with the specificity of SiaDW-135 for galactose H4 Gal received 57% saturation and, remarkably, also the opposite site of the pyranose ring, the anomeric proton H1 Gal, received considerable saturation (62%). For experimental details, see Fig. 6.