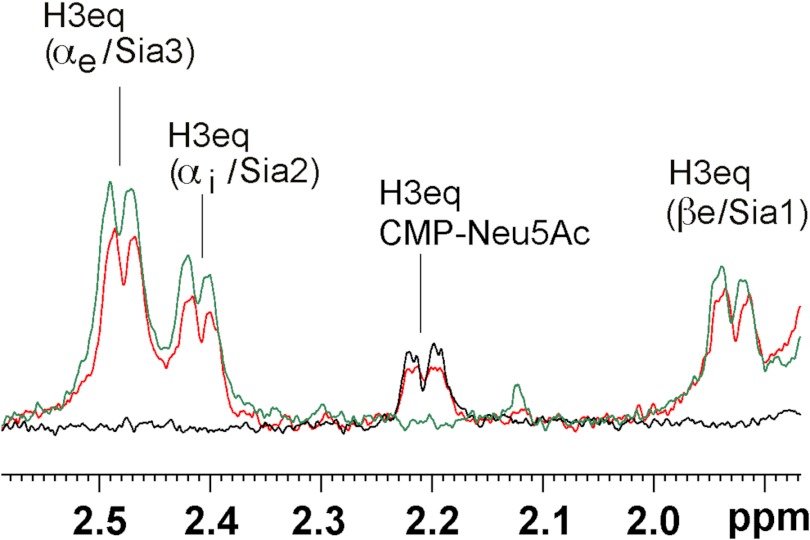
FIGURE 9.
STD NMR spectra of SiaDW-135 in complex with CMP-Neu5Ac and the acceptor DP3. To evaluate if binding of the acceptor DP3 influences the binding of CMP-Neu5Ac, three STD NMR spectra were overlaid. In black and green the spectra obtained for SiaDW-135 in complex with CMP-Neu5Ac and DP3, respectively, are shown. The spectrum obtained when both substrates were present in equimolar concentrations is shown in red. The absolute STD NMR intensities revealed only a minor (∼15%) reduction in the saturation transfer to the H3eq proton of CMP-Neu5Ac when DP3 was present. Similarly, a reduction of ∼20% was seen for the H3eq protons of DP3 under these conditions. The faintness of the differences observed in the presence of the second substrate argued for independent binding sites. For experimental details, see Fig. 6.