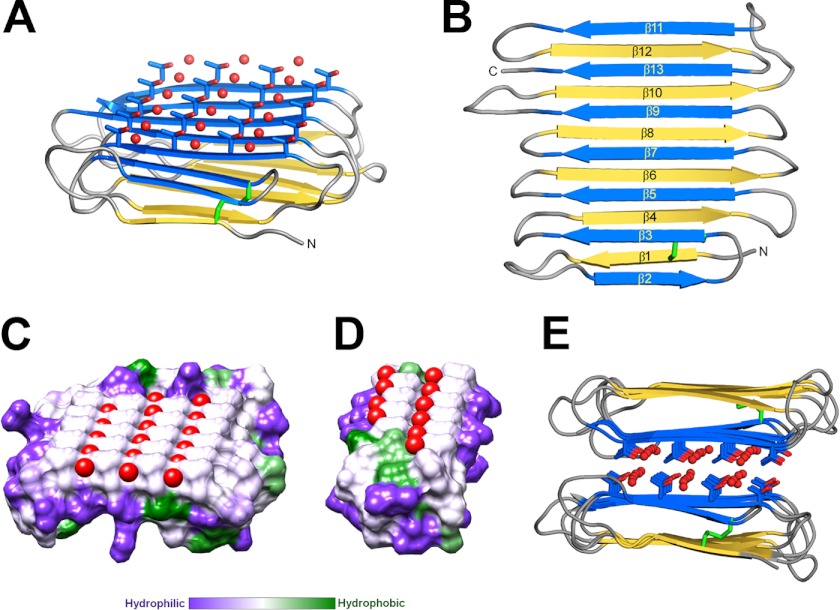
FIGURE 2.
Crystal structure of RiAFP. A, overall fold of RiAFP. β-Strands are shown in blue and yellow. The threonine side chains and coordinated water molecules (red) on the IBS are shown in ball-and-stick representation. The disulfide bond is indicated in green. B, secondary structure diagram with sequentially numbered ice-binding β-strands in blue, hydrophilic β-strands in yellow. N and C represent the protein termini. C and D, flat ice-binding surface of RiAFP (C) and TmAFP (D), represented by a hydrophobic surface diagram produced in Chimera, which relies on the Kyte-Doolittle scale to rank amino acid hydrophobicity. Water molecules coordinated by the threonine hydroxyls on the IBS are shown in red. E, RiAFP dimers in the crystallographic asymmetric unit, with the ice-binding surfaces packed face-to-face.