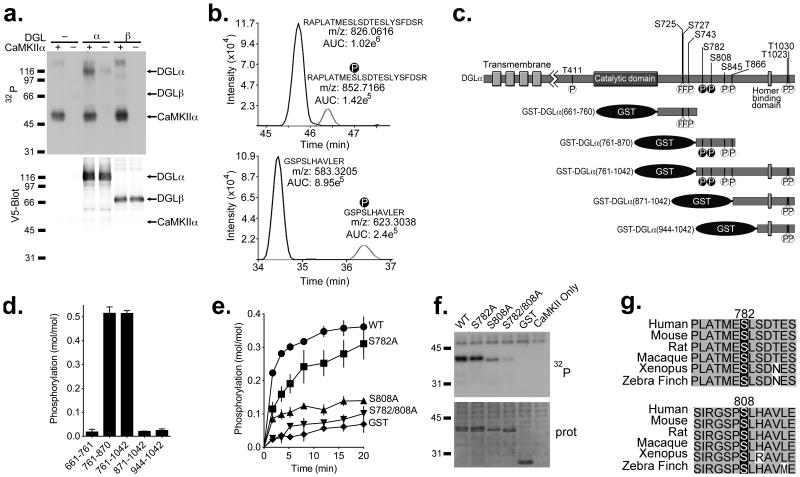
Figure 2. CaMKIIα selectively phosphorylates DGLα at Ser782 and Ser808.
(a) Purified DGLα/β-V5 was incubated with [γ-32P]ATP and recombinant CaMKIIα and analyzed by SDS-PAGE. The ~116 kDa 32P-labeled band on the autoradiograph (32P) overlaps with the DGLα-V5 band shown on the corresponding V5 western blot (Blot: V5), and was not detected in the absence of CaMKIIα or DGLα-V5. DGLβ-V5 was not significantly 32P-phosphorylated. (b) Extracted ion chromatograms are shown for phosphorylated and non-phosphorylated DGLα peptides containing Ser782 and Ser808. The monoisotopic m/z values for the observed [M+3H]3+ and [M+2H]2+ precursor ions for DGLα peptides 774–795 (top) and 805–815 (bottom), respectively, are provided adjacent to their corresponding chromatographic peak. Observed values are within 2 ppm of theoretical values calculated for these precursor ions. (c) Phosphorylation sites identified by mass spectrometric analysis of full length WT DGLα purified from HEK293 cells are indicated (see Table 1). The two CaMKII-specific sites, Ser782 and Ser808 are indicated with black circles. GST-fusion proteins used in panel d are also shown. (d) GST-fusion proteins containing C-terminal tail fragments of DGLα were incubated with recombinant CaMKIIα and [γ-32P]ATP. CaMKII selectively phosphorylated fragments containing amino acids 761–870. (e) Time course for phosphorylation of GST-DGLα (761–870) (WT, S782A, S808A or S782A/S808A) or GST alone by CaMKIIα. (f) Representative autoradiograph (32P) and protein stain (prot) of SDS-PAGE analysis of reactions from panel (e) at 10 min. (g) Ser782 and Ser808 are conserved in a range of vertebrates.