Figure 1. Study design and timelines.
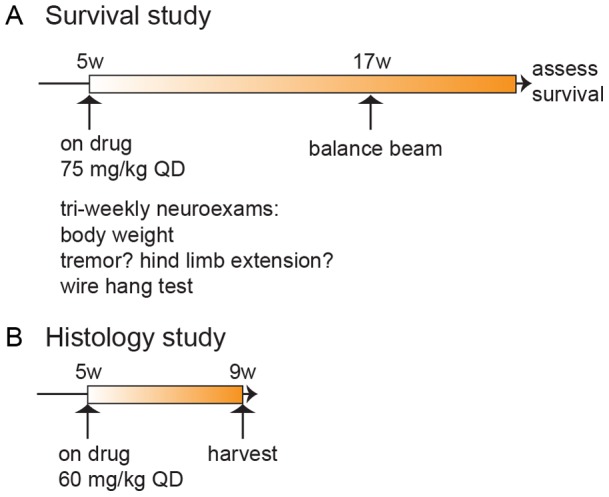
(A) Study 1: timeline for survival study. n = 106 mice (see Table 1 for breakdown of n per genotype and treatment) received daily IP doses of erlotinib from 5 weeks of age onwards, and their lifespan was measured. Mice were assessed in neurological exams 2 times/week between 6–9 weeks, 3 times/week from 9 weeks of age onwards, and tested on the balance beam at 17 weeks of age. (B) Study 2: timeline for histological endpoints study. Mice (n = 34; see Table 1 for breakdown by genotype and treatment) were dosed from 5–9 weeks of age, and euthanized for tissue collection at 9 weeks.
