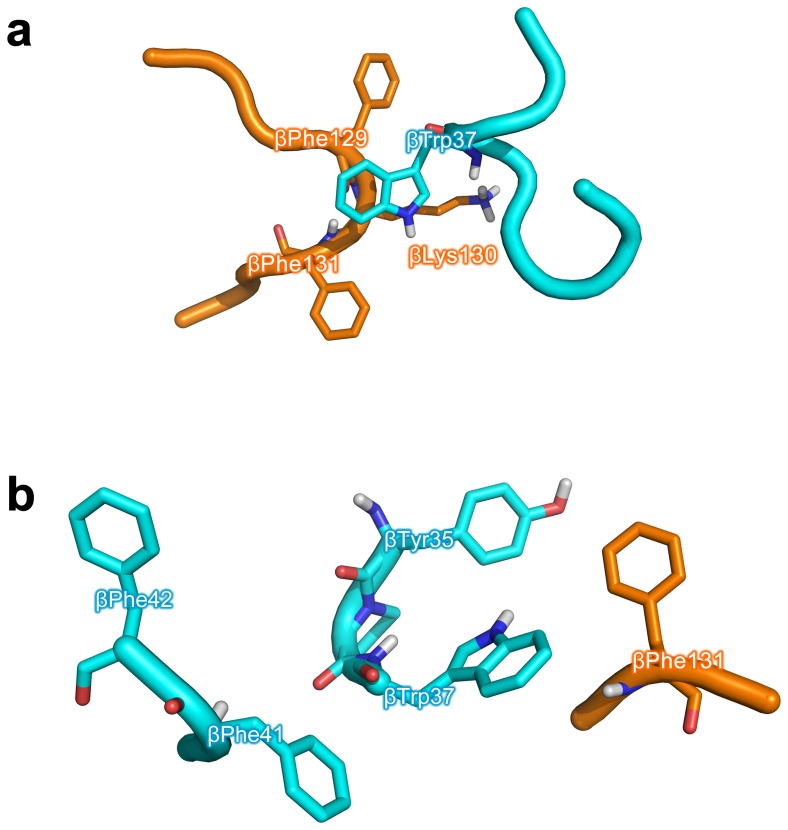
Figure 7. Molecular modeling analysis at the binding interface of the top ranked complex of Hb-Hp with particular focus on βTrp37 of Hb (a) and βPhe131 of Hp (b).
The former panel shows βTrp37 of Hb participating in π-π ring stacking interaction with two neighboring phenylalanines of Hp, βPhe129 and βPhe131, as well as engaging in π-cation interaction with a nearby lysine of Hp, βLys130. The latter panel shows βPhe131 of Hp taking part in intermolecular π-π stacking interactions with aromatic residues of Hb, βTyr35 and βTrp37. In addition to the inner sphere of aromatic residues of Hb (comprising of βTyr35 and βTrp37), a second outer sphere of aromatic residues of Hb (comprising of βPhe41 and βPhe42) is also present.