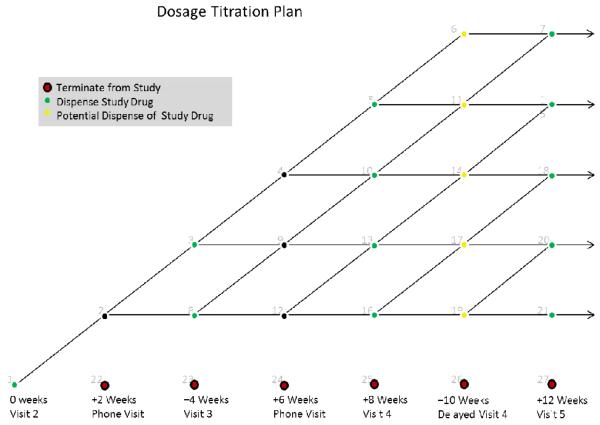
Figure 2.
Titration Plan. Subjects will start titration at randomization visit (visit 2, 0 weeks of medication). At 2 week intervals, subjects will be assessed for tolerability of medication treatment. At each of these nodal points, an assessment is made as to whether to continue increasing the titration, holding the current dose, returning to the previous dose (i.e, dose reduction). All choices are available at each nodal point. However, if 2 reduction are made, subjects will be terminated from the study and proceed to visit 8 assessment. If there is a dosage hold or reduction, subjects will be allowed to have an additional 2 weeks of titration in order to maximize dosing.