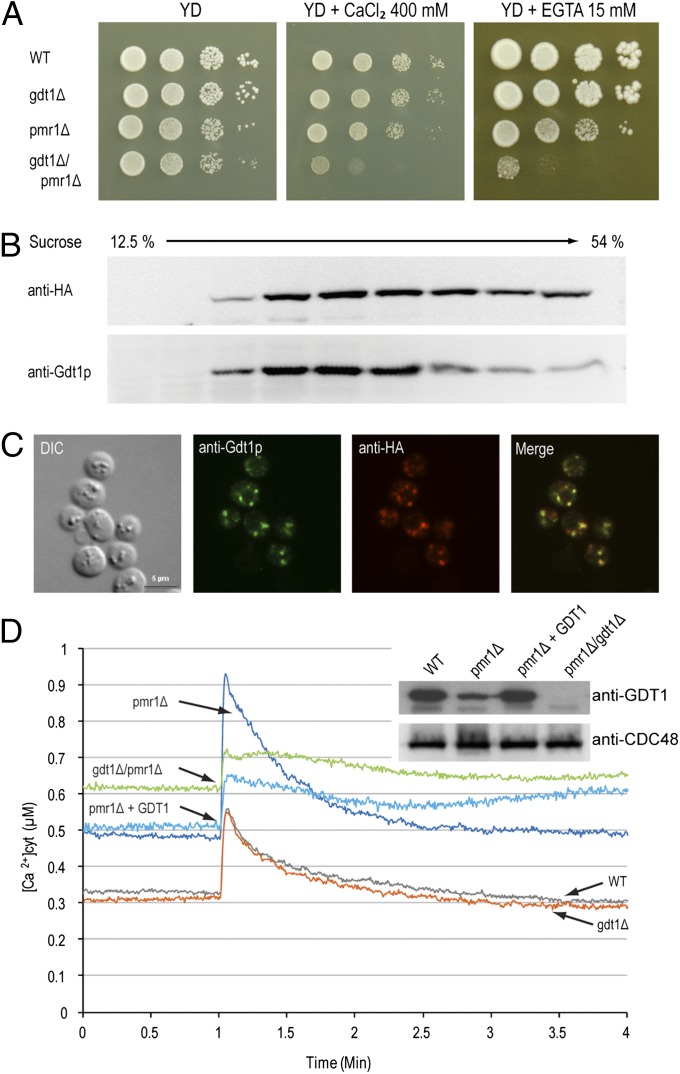
Fig. 1.
GDT1 genetically interacts with PMR1. (A) The different mutants were precultured in YD medium to an OD600 of 0.3, then serial 10-fold dilutions were dropped onto solid YD medium alone or supplemented with 400 mM CaCl2 or 15 mM EGTA and the plates incubated at 28 °C for 4–6 d. Colocalization of Gdt1p and HA-Pmr1p as shown by subcellular fractionation (B) or immunostaining (C). (B) The fractions collected from the top of a discontinuous 10-step sucrose gradient were analyzed by Western blotting using antibodies against HA-Pmr1p (anti-HA) or Gdt1p. (C) Gdt1p and HA-Pmr1p in yeast were labeled using rabbit or rat primary antibodies, respectively, and fluorescein-conjugated anti-rabbit IgG and Alexa Fluor 546–conjugated anti-rat IgG antibodies. Differential interference contrast (DIC) and merged (merge) pictures are also presented. (Scale bar, 5 µm.) (D) The different strains expressing apo-aequorin from a plasmid were incubated overnight in minimal medium containing coelenterazine to reconstitute the holoenzyme. When the OD600 reached 3.0, 200 µl of each culture was transferred to a luminometric tube and the baseline luminescence monitored for 60 s, then CaCl2 was injected to a final concentration of 133 mM and the signal monitored for 3 min. The [Ca2+]cyt values were derived from luminometric units using the equation from Allen et al. (1977) (37). All displayed results are representative of those seen with at least three replicates. (Inset) Gdt1p and Cdc48p (loading control) levels in the different mutants measured by Western blotting of total extracts using anti-Gdt1p or anti-Cdc48p antibody. “pmr1Δ + GDT1” corresponds to the pmr1Δ mutant overexpressing GDT1.