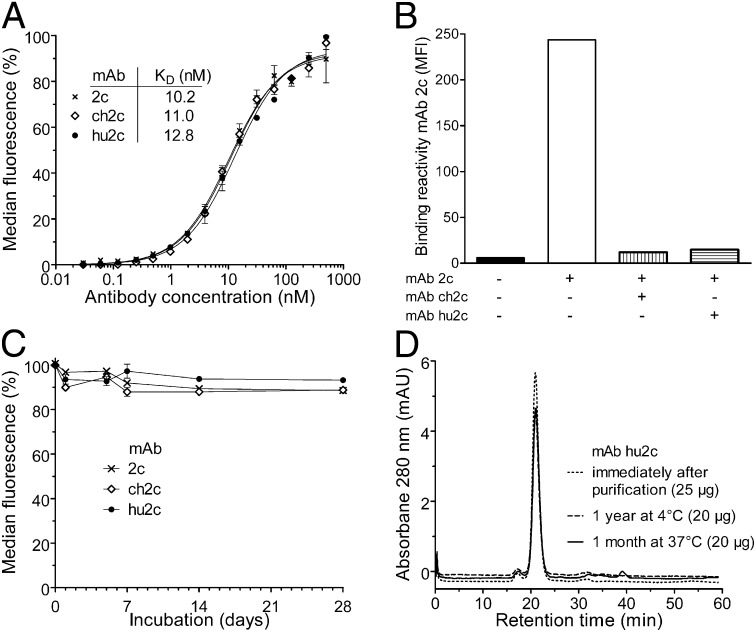
Fig. 1.
Humanized mAb hu2c retains the same specificity and biophysical properties of its parental murine mAb 2c. (A) Equilibrium-binding curves of mAbs 2c, ch2c, and hu2c as determined by flow cytometric analysis with HSV-1 infected Vero cells. (B) Binding of murine mAb 2c to glycoprotein B on the surface of HSV-1 F-infected Vero cells could be blocked either by mAb ch2c or mAb hu2c at 10-fold molar excess. Flow cytometric measurements are representative for at least two experiments performed in triplicate ± SEM. (C) Biological stability. Antigen-binding activity of antibodies (15 µg/mL, PBS) was assayed by flow cytometry after incubation for various time periods at 37 °C. (D) Same batch analysis of purified mAb hu2c (1 mg/mL in PBS) either stored at 4 °C for 1 y or 37 °C for 1 mo by size exclusion FPLC on a Superdex 200 column revealed single monomeric peaks with elution times (21 ± 0.07 min) identical to the profile of mAb hu2c analyzed immediately after production and purification.