Abstract
Objective
Ureaplasma colonization in the setting of polymicrobial flora is common in women with chorioamnionitis, and is a risk factor for preterm delivery and neonatal morbidity. We hypothesized that ureaplasma colonization of amniotic fluid will modulate chorioamnionitis induced by E. coli lipopolysaccharide (LPS).
Methods
Sheep received intra-amniotic (IA) injections of media (control) or live ureaplasma either 7 or 70d before delivery. Another group received IA LPS 2d before delivery. To test for interactions, U. parvum exposed animals were challenged with IA LPS, and delivered 2d later. All animals were delivered preterm at 125±1 day gestation.
Results
Both IA ureaplasmas and LPS induced leukocyte infiltration of chorioamnion. LPS greatly increased the expression of pro-inflammatory cytokines and myeloperoxidase in leukocytes, while ureaplasmas alone caused modest responses. Interestingly, 7d but not 70d ureaplasma exposure significantly downregulated LPS induced pro-inflammatory cytokines and myeloperoxidase expression in the chorioamnion.
Conclusion
Acute U. parvum exposure (7d) can suppress LPS induced chorioamnionitis.
Keywords: Endotoxin tolerance, Preterm labor, Innate immunity, Fetal adaptation
Introduction
Premature birth is a major national health concern as it contributes disproportionately to neonatal morbidity and mortality, accounting for 12.3% of live-births and >60% of neonatal deaths in the United States 1. Both clinical and experimental studies strongly demonstrate a causal link between intrauterine infection and preterm labor 2, 3. The most common organisms isolated from the amniotic fluid of women with chorioamnionitis are the Ureaplasma species 4. Furthermore, among the very early preterm infants with chorioamnionitis caused by the Ureaplasma spp., about 60% also had co-infection with other organisms 4. Exposure of preterm infants to ureaplasmas is associated with increased risk for adverse pulmonary, gastrointestinal and neurological outcomes 5.
Despite the reports demonstrating adverse outcomes, ureaplasma infection often is silent clinically. Ureaplasma spp. have been isolated in amniotic fluid samples from genetic amniocenteses done at 15–21 weeks gestation 6, 7. Although the rates of preterm deliveries were higher in women with second trimester Ureaplasma spp. in the amniotic fluid, the majority of the women delivered at term gestation. The reasons why some pregnancies with chronic ureaplasma infection result in preterm delivery while others deliver at term are unknown. There is minimal information on modulation of innate immune system of the maternal or fetal host by the Ureaplasma spp.
We reported that intraamniotic injection of U. parvum in sheep induced a chronic colonization with poor host clearance of the organism from the amniotic fluid 8. Interestingly, chronic but not acute infection of amniotic fluid with ureaplasmas suppressed E. coli endotoxin (LPS) induced inflammation in the fetal lung, indicating innate immune tolerance 9. Further, we previously demonstrated that multiple exposures to intraamniotic LPS also induced endotoxin tolerance in the preterm fetus 10, 11. We therefore hypothesized that chronic but not acute exposure to ureaplasmas will down-regulate LPS induced chorioamnionitis. We report inflammatory responses in the chorioamnion to intraamniotic exposures to acute and chronic ureaplasmas, to LPS and the interactions between ureaplasma and LPS exposures.
Materials and Methods
Animals and Intra-Amniotic Injections
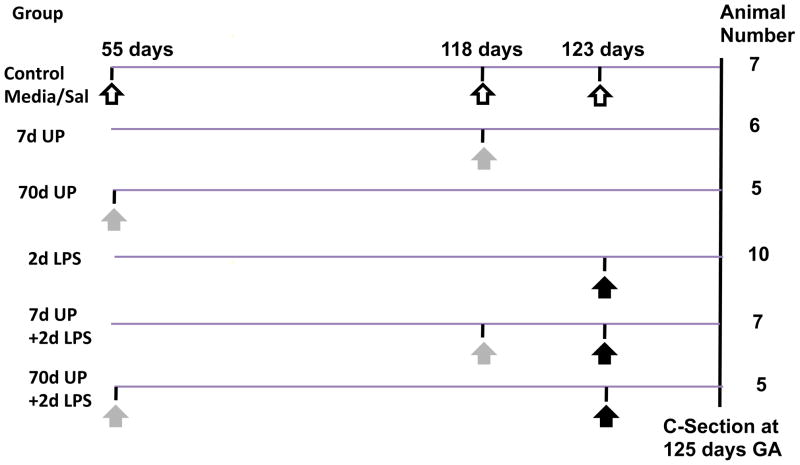
All animals were studied in Western Australia with approval from the animal ethics/care and use committees of the Cincinnati Children’s Hospital Medical Center (Cincinnati, OH) and the University of Western Australia. The fetal pulmonary data from these animals were previously reported 9. Time-mated Merino ewes with singleton fetuses were randomly assigned to six groups of 5 to 10 animals (Figure 1). All injections were given by the intra-amniotic (IA) route under ultrasound guidance, and after verification of proper placement in the amniotic compartment by fluid electrolyte analysis of aspirated samples 12. Sheep received an IA injection of 1) U. parvum serovar 3 (2 × 107 colony forming units) or 2 mL media as a control 70 days or 7 days prior to delivery, 2) E. coli LPS (O55:B5; Sigma-Aldrich, St. Louis, MO) 10 mg in 2 mL saline or saline only as a control 2 days prior to delivery. To evaluate the interactions, sheep were given IA injections of U. parvum 7d or 70d prior to delivery plus IA LPS 2 days before delivery. Animals were delivered by cesarean section at 125 ± 1 day gestational age (term ewe gestation = 150 days). Fetuses were given lethal intravascular doses of pentobarbital for euthanasia. At the time of delivery, rolls of fetal chorioamnion membranes were snap-frozen for RNA analysis, and a roll was fixed in 10% buffered formalin (pH 7.4) for histology. A fresh sample of amniotic fluid was used for pH measurement. Amniotic fluid was also snap-frozen for measurement of cytokine proteins. Ureaplasma spp. within sheep chorioamnion were confirmed by culture of homogenized tissue in 10B broth medium using previously reported protocols 13, 14.
Figure 1. Schematic of the experimental design.
The different study groups with the interventions along with the sample size is shown. All injections were given by the intraamniotic route and the timing in gestational days is shown. Open arrow indicates either media or saline, grey arrow indicates U. parvum and black arrow indicates LPS injection. (Term gestation=150d in sheep).
Immunohistochemistry and Scoring of Inflammation
Inflammatory cells in the chorioamnion were quantified by immunohistochemistry for markers of inflammatory cell activation using 5 μm paraffin sections from formalin fixed chorioamnion. The sections were de-paraffinized and rehydrated, and antigen-retrieval was carried out using citric acid buffer, pH 6.0, with boiling in a microwave. Endogenous peroxidase activity was blocked with methyl alcohol/hydrogen peroxide. Nonspecific binding was inhibited with 2% goat serum during both primary and secondary antibody incubation. Sections were incubated overnight at 4°C with anti-myeloperoxidase (MPO) antibody (1:400) (Cell marque, Rocklin, California, catalogue # CMC028), or guinea-pig polyclonal anti-monocyte chemoattractant protein (MCP)-1 antibody (1:1000) generated at our institution (Seven Hills Bioreagents, Cincinnati, OH). After washing, sections were incubated with the appropriate secondary antibody for 30 minutes at room temperature (1:200). Immunostaining was visualized using a Vectastain ABC peroxidase Elite kit abd enhanced with nickel-diaminobenzidine, followed by incubation with TRIS-cobalt to give a black precipitate. Nuclei were counterstained with Nuclear Fast Red for photomicroscopy. Scoring of MPO and MCP-1 positive cells in 10 comparable non-overlapping high-power fields for each animal was done by a single investigator blinded to study groups. For qualitative scoring of chorioamnionitis, the sections were stained with hemotoxylin and eosin, and staged and graded for degree of inflammation in a blinded manner using a modified Redline grading system 15. Scores denoting the magnitude and tissue distribution of neutrophil infiltration within the chorioamnion were reported as follows: Score 0= No chorioamnionitis, Score 1 = Stage 1, Grade 1 chorioamnionitis, Score 2 = Stage 1, Grade 2 chorioamnionitis, Score 3 = Stage 2, Grade 2 chorioamnionitis, and score 4 = Stage 3, Grade 2 chorioamnionitis. In this scoring system, the stage of chorioamnionitis denotes the tissue plane of inflammation and the grade denotes the severity of inflammation. Stage 1 chorioamnionitis refers to inflammation restricted to the choriodecidua, Stage 2 is inflammation in the choriodecidua + amnion and Stage 3 denotes necrosis of the amniotic epithelium.
Cytokine mRNA Quantitation
Total RNA was isolated from the frozen chorioamnion samples using a modified Chomczynski method 9. The quantitation of mRNA was performed by real-time polymerase chain reaction (PCR) after reverse transcription of the mRNA yielding a single-strand complementary DNA (cDNA) (Verso cDNA kit, Thermo Scientific, Waltman, MA). cDNA was then used as a template with primers and TaqMan probes (Applied Biosystems, Carlsbad, CA) specific to sheep sequences for interleukin (IL)-1β, IL-6, IL-8, IL1-RA, MCP-1 and serum amyloid A3 (SAA3) 16. The values for each cytokine were normalized to the internal 18S ribosomal RNA (rRNA) value. Final expression data are represented as fold increase over the control value.
Amniotic Fluid Protein Analysis
Cytokine protein quantification was performed using custom sandwich enzyme-linked-immunosorbent assay (ELISA) 17, 18. The following antibody sets were used: 1) IL-1β [coating Ab: rabbit anti-ovine IL-1β and primary Ab guinea-pig anti-ovine IL-1β (Seven Hills Bioreagents)], and 2) IL-6 [coating Ab: mouse anti-ovine IL-6 (Chemicon # MAB1004) and primary Ab rabbit anti-ovine IL-6 (Chemicon #AB1839)]. The detection antibody in all assays was an appropriate species-specific HRP-conjugated antibody. The dynamic range of detection was 0.2–12 ng/mL for IL-1β and IL-6. The correlation coefficient was 0.94–0.99 for all assays.
Data Analysis
Results are reported as mean ± SEM. All comparisons were specified a priori. Comparisons among three or more groups were performed by Kruskal-Wallis non-parametric analyses of variance (ANOVA). Comparison of two groups was done by a nonparametric t test (Mann-Whitney U test) for data not normally distributed (GraphPad Prism, version 5.04 for Windows La Jolla, California). The control group was compared versus 7d UP, 70d UP, and 2d LPS. To specifically examine the effect of U. parvum infection on LPS response, 2d LPS was compared versus 7d UP+ 2d LPS, and 70d UP + 2d LPS. Statistical significance was accepted at p<0.05.
Results
Intra-amniotic infection with U. parvum did not cause any gross fetal developmental abnormalities or mortality. Consistent with ureaplasma utilizing urea as a nutritional source with liberation of ammonia, the amniotic fluid pH values differed significantly among groups (p=0.01) by ANOVA (Figure 2). By post-hoc analysis, the only group with a higher pH compared to control was the 70 day U. parvum group (the 70d UP+ 2d LPS group (last bar in Figure 2) had only two animals and hence statistical difference could not be established.
Figure 2. Chronic U. parvum infection increases amniotic fluid pH.
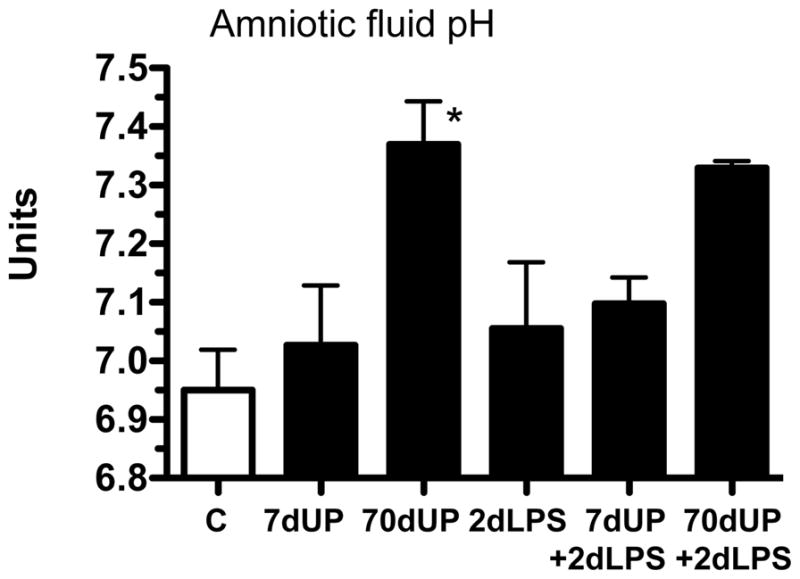
The pH of the amniotic fluid was measured at autopsy. 70d exposure but not 7d exposure to U. parvum increased the pH of amniotic fluid (*p< 0.05 versus controls).
Histological chorioamnionitis after intra-amniotic injections of LPS and U. parvum
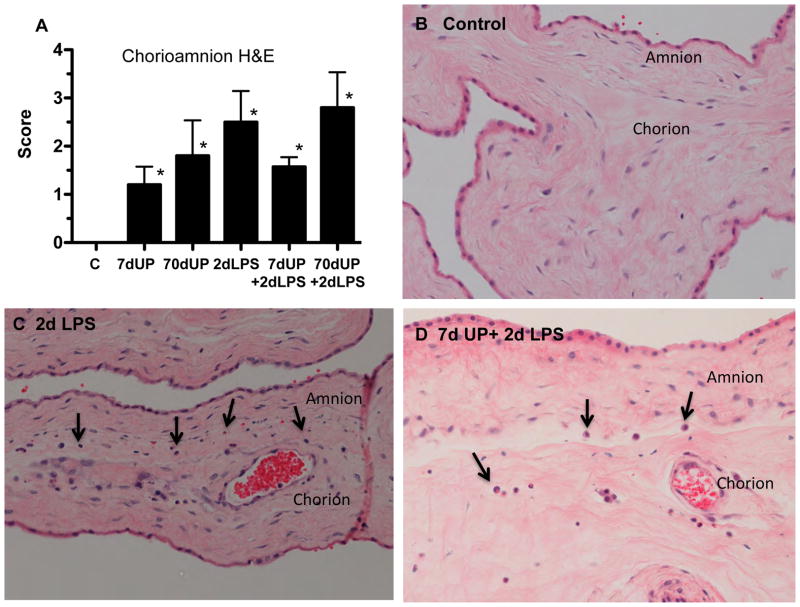
To evaluate the degree of histologic chorioamnionitis among groups, the infiltration of neutrophils in the chorioamnion were scored qualitatively (Figure 3). Relative to the control, all treatment groups had significantly increased recruitment of neutrophils and higher scores for chorioamnionitis (p=0.004), but there were no differences between the LPS and U. parvum groups.
Figure 3. Staging of Chorioamnionitis after intra-amniotic injections of LPS and U. parvum.
(A) Blind scoring for severity of chorioamnionitis was performed using a modified Redline staging method. Representative photomicrographs showing hematoxylin and eosin staining of chorioamnion sections from (B) controls, (C) 2 day LPS, and (D) 7 day U. parvum + 2 day LPS. Arrows point to inflammatory cells at the junction of amnion and chorion (*p< 0.05 versus controls).
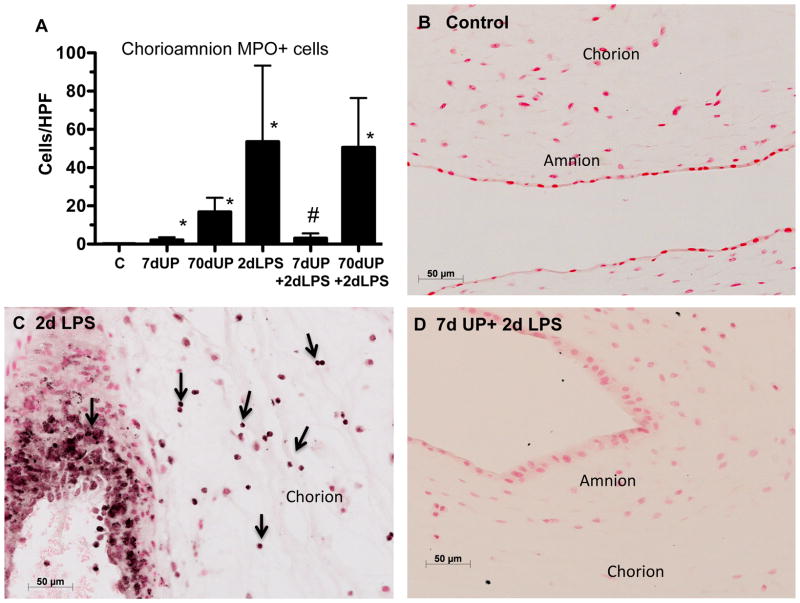
To further evaluate the activation of inflammatory cells in the chorioamnion, myeloperoxidase (MPO) in the inflammatory cells was quantified (Figure 4). Relative to controls, MPO positive cells significantly increased in all groups except in those sheep with acute U. parvum + LPS exposure. Compared to LPS exposure alone, MPO positive cells significantly decreased in the 7 day U. parvum + LPS group, but not after chronic U. parvum exposure (70d) and LPS challenge.
Figure 4. Acute amniotic exposure to U. parvum decreased intra-amniotic LPS-induced myeloperoxidase (MPO) expression.
(A) Quantitation of MPO-positive cells per microscopic high-power field is shown. Representative photomicrographs are shown for MPO immunostaining in (B) controls, (C) 2 day LPS, and (D) 7 day U. parvum + 2 day LPS. Arrows point to inflammatory cells in the chorion in panel C (*p< 0.05 versus controls. #p< 0.05 versus 2 day LPS).
Acute exposure to U. parvum down-regulated LPS-induced cytokine expression in the chorioamnion/amniotic fluid
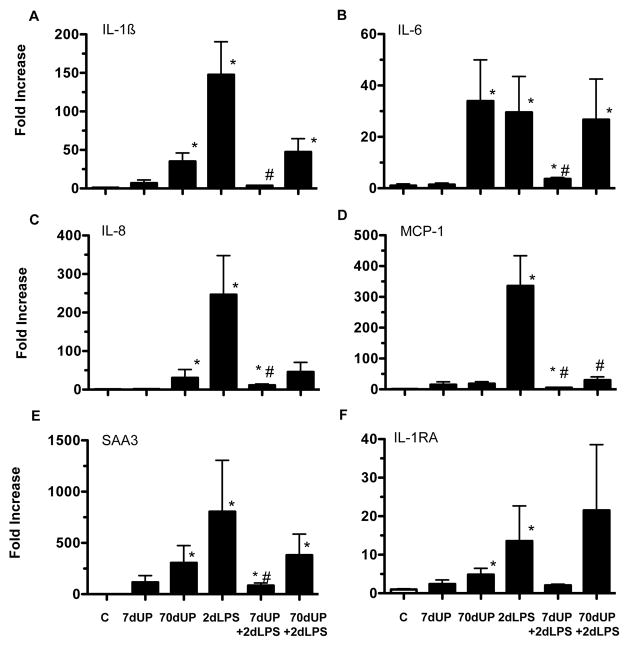
We previously demonstrated that the chorioamnion is a target of inflammation after exposure to intraamniotic (IA) administration of pro-inflammatory agonists 19. We therefore measured expression of pro-inflammatory cytokine, chemokine, acute phase reactant and anti-inflammatory cytokine mRNAs in the chorioamnion (Figure 5A–F). Relative to controls, acute (7 d exposure) did not increase cytokine mRNA expression while chronic (70 d exposure) to U. parvum induced increases in mRNA expression for IL-1β (35 fold), IL-6 (34 fold), IL-8 (31 fold), SAA3 (300 fold), and IL-1RA (5 fold). The responses in the 70d UP + 2d LPS were similar to 70d UP alone (p=0.6–0.8 for different cytokines for 70d UP vs. 70d UP+2d LPS). In contrast, exposure to IA LPS for 2 d greatly increased expression of both the pro-inflammatory cytokines/chemokines: IL-1β (148 fold), IL-6 (30 fold), IL-8 (246 fold), MCP-1 (336 fold), SAA3 (800 fold), and the anti-inflammatory cytokine IL-1ra (14 fold). Prior exposure to U. parvum for 70 d did not consistently change responses to LPS (MCP-1 mRNA decreased compared to LPS alone, while IL-1β, IL-6, IL-8, SAA3 and IL-1RA did not). In sharp contrast, acute exposure to U. parvum (7d), significantly and consistently down-regulated LPS responses to near control levels. The cytokine expression in the 7d UP + 2d LPS group were similar to the 7d UP alone with the exception of IL-8 (p=0.04) and IL-6 (p=0.07).
Figure 5. Acute exposure to U. parvum down-regulated LPS-induced cytokine mRNA expression in the chorioamnion.
Quantification of messenger RNAs for (A) IL-1β, (B) IL-6, (C) IL-8, (D) MCP-1, (E) serum amyloid A3 and (F) IL-1 receptor antagonist was performed by real-time PCR assays using sheep-specific primers and TaqMan probes. Each cytokine was normalized to 18S ribosomal protein mRNA (internal control), and levels for each group were expressed relative to controls as fold increase relative to controls. (*p< 0.05 versus controls, #p< 0.05 versus 2 day LPS).
To further confirm these findings, we measured IL-1β and IL-6 protein levels in the amniotic fluid. Neither acute nor chronic exposure to U. parvum alone increased secretion of IL-6 (Figure 6A) or IL-1β (Figure 6B) in the amniotic fluid. Consistent with the mRNA responses, IA LPS increased IL-1β and IL-6 secretion. Similar to the mRNA effects, acute but not chronic prior exposure to U. parvum decreased LPS mediated induction of IL-6. There were non-significant trends to decrease LPS induced IL-1β expression with prior acute (p=0.3) or chronic (p=0.06) U. parvum exposure.
Figure 6. Acute exposure to U. parvum down-regulated LPS-induced cytokine proteins in the amniotic fluid.
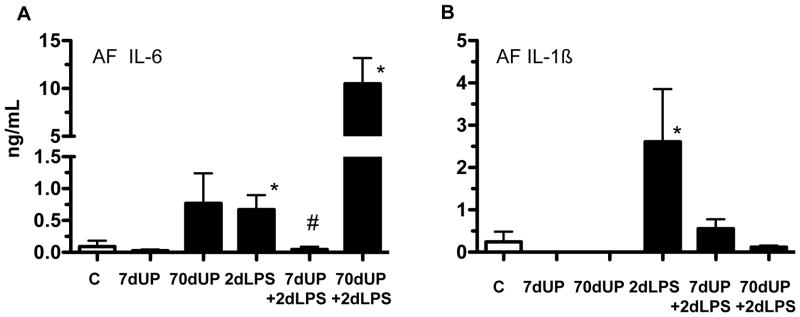
Cytokine proteins in the amniotic fluid were measured by ELISA, (A) IL-6 and (B) IL-1β. (*p < 0.05 versus controls. #p < 0.05 versus 2 day LPS).
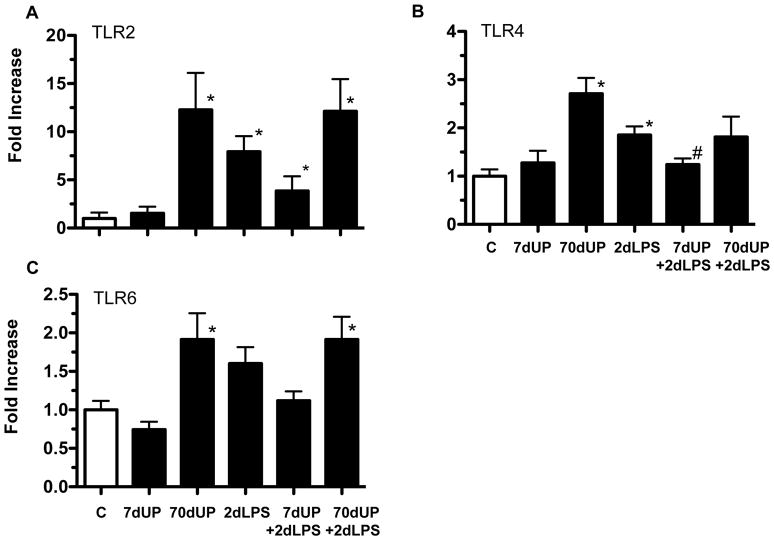
To begin to get mechanistic insights, we measured the mRNA expression of the Toll-like receptors in the chorioamnion that signal LPS responses (TLR4) and U. parvum responses (TLR2 and TLR6) 20. The chronic but not acute exposure to U. parvum increased expression of TLR2 (12 fold), TLR4 (3 fold) or TLR6 (2 fold) (Figure 7A–C). LPS alone also increased expression for TLR2 (8 fold) and TLR4 (2 fold). Interestingly, compared to the 2d LPS group, the TLR4 mRNA expression did not increase in the 7d UP+2d LPS group. In contrast, there were no significant differences in the TLR expression between the 2d LPS group vs. 70d UP+2d LPS group (p=1.0 for TLR2, p= 0.2 for TLR4, and p=1.0 for TLR6). Compared to the 7d UP group, only TLR6 mRNA expression was higher in the 7d UP + 2d LPS group (p=0.05).
Figure 7. Acute exposure to U. parvum down-regulated LPS-induced TLR mRNA expression in the chorioamnion.
Quantification of messenger RNAs for (A) TLR2, (B) TLR4, and (C) TLR6 was performed by real-time PCR assays using sheep-specific primers and TaqMan probes. Each cytokine was normalized to 18S ribosomal protein mRNA (internal control), and levels for each group were expressed relative to controls as fold increase relative to controls. (*p< 0.05 versus controls, #p< 0.05 versus 2 day LPS).
Discussion
We report a novel finding that Ureaplasma spp. infection of the amniotic cavity can profoundly suppress signaling by LPS, a constituent of the cell wall of Gram-negative bacteria. Microbial invasion of amniotic cavity is relatively unique among infectious diseases in humans: The microbes responsible are mostly opportunistic pathogens in otherwise healthy women, polymicrobial growth is common, and Ureaplasma spp. that rarely cause infections elsewhere are the most common organism 21, 22. The interactions between Ureaplasma spp. and other microbial products in this polymicrobial milieu have not been studied. Adding to the intrigue, chorioamnionitis has been associated with variable outcomes ranging from no pathology to the mother or the fetus to significant morbidity to the mother/fetus 5, 23, 24. Our results offer a possible explanation for the variable inflammation in chorioamnionitis in the setting of multiple microbial products in the amniotic cavity.
Endotoxin tolerance resulting in suppressed LPS signaling causes a complex re-programming of inflammatory responses 25. Pro-inflammatory cytokine expression is down regulated, while there is no change or even an increase in the expression of anti-inflammatory genes, antimicrobial genes, genes mediating phagocytosis 25. The net result of endotoxin tolerance appears to prevent host organ injury while maintaining antimicrobial functions. However, almost all the information regarding endotoxin tolerance is derived from gene expression studies in vitro in functionally mature monocytes or macrophages. The genes reported not to be down regulated after endotoxin tolerance include IL-10, IL-1RA, TGFβ1, serum amyloid and others 26. In our study, the upregulation of TLR4 expression after IA LPS exposure was prevented by prior exposure to a 7d U. parvum exposure. IL-1ra and serum amyloid A3 gene were also down regulated similar to the other pro-inflammatory cytokines in the acute (7d) U. parvum +LPS exposed fetuses. Similarly, the activation of inflammatory cells as indicated by myeloperoxidase expression also decreased in the acute (7d) U. parvum +LPS fetuses, relative to the LPS alone exposed animals. These results suggest that acute exposure to U. parvum induced down regulation of LPS responses were more global in nature than previously reported in endotoxin tolerance in vitro 26 and could be partially modulated by changing the toll-like receptor gene expression.
Since endotoxin tolerance is an adaptive host response, the characteristics of endotoxin tolerance might vary depending on the inflammatory context. We previously reported that preterm sheep fetuses exposed to repeated doses of intraamniotic LPS demonstrate endotoxin tolerance and cross-tolerance to other Toll-like agonists 10, 27. Further, we recently reported that chronic (70d) but not acute (7d) exposure to U. parvum suppressed LPS signaling in the fetal lung. In contrast to the lung findings and our hypothesis, we found a consistent suppression of LPS induced inflammatory responses in the chorioamnion of the acute (7d) ureaplasma exposed animals. Interestingly, relative to the LPS alone group, the chronic (70d) + LPS group had an inconsistent suppression of LPS induced pro-inflammatory cytokine gene expression. The mechanisms of the different time window of tolerance like responses in different fetal tissues are not known. Manimtim et al 28 reported that lower doses of ureaplasmas inhibited LPS induced IL-6 secretion by blood monocytes from preterm infants in in vitro studies, while higher doses augmented LPS responses. These divergent responses may be due to the in vivo responses of the chorioamnion, while studies by Manimtim et al were in vitro experiments using blood monocytes. Taken together, these experiments demonstrate that ureaplasmas can modulate LPS responses in a time and tissue dependent manner.
The gold standard for diagnosis of chorioamnionitis is histological evidence of inflammatory cell infiltration 15. However the organ injury is caused by products of inflammation e.g. cytokines and prostaglandins. Therefore, the pathophysiology of inflammation-induced damage requires recruitment of inflammatory cells, followed by activation and elaboration of products of inflammation. Interestingly, our data demonstrate that exposure to ureaplasmas alone or LPS exposure alone induced histological chorioamnionitis. However, despite the presence of histological chorioamnionitis, relative to the LPS only group, the 7d ureaplasma + 2d LPS exposure group had suppressed cytokine expression and very few myeloperoxidase expressing cells in the chorioamnion, indicating that the inflammatory cells were not activated. These results demonstrate that ureaplasmas selectively suppress LPS induced inflammatory cell activation in the chorioamnion but not cell recruitment.
Microbial load has been postulated to be a determinant of the severity of chorioamnionitis 29. However, we previously reported that in sheep whose amniotic cavity was inoculated with U. parvum serovar 6, there was a chronic colonization with poor clearance of the organism that persisted for more than 12 weeks post inoculation 8. Consistent with our previous results demonstrating chronic colonization, we observed in these animals, no significant differences in the colony counts of ureaplasmas 7d or 70d after intramniotic injection of 2 × 107 CFU 9. Nevertheless, chronic U. parvum infection increased the pH of the amniotic fluid, while acute infection did not. The increase in pH generated by ureaplasmas within amniotic fluid may limit the numbers of CFUs within the body fluid just as ureaplasma growth in vitro is limited by the generation of ammonia as an end product of metabolism. Therefore increased amniotic fluid pH may serve as a biomarker of chronicity of U. parvum infection. In the present study, there was minimal inflammation in the chorioamnion with 7d exposure to ureaplasmas, but with 70d chronic exposure there was a modest but discernable increase in pro-inflammatory cytokine expression and myeloperoxidase expression in the chorioamnion.
Recent PCR based methods supplementing cultures have identified a host of infectious agents isolated from the amniotic fluid of women in preterm labor 21. Apart from Ureaplasma and Mycoplasma species, Gram-negative organisms (Fusobacterium, Sneathia, Bacteroides, Prevotella spp.) and Gram positive organisms (Streptococcus and Staplylococcus spp.) were identified from the amniotic fluid of these women 21. We previously reported that repeated exposures to intraamniotic LPS induces cross-tolerance to other toll-like agonists in preterm sheep 27. Whether our findings of suppression of E. coli LPS responses by Ureaplasma spp. extend to other organisms (signaling via different Toll-like receptors) remains unknown. Similarly the generalizability of endotoxin tolerance response across different gestation ages, and duration of Ureaplasma exposures await further study. Also, the effects of chronic exposure to LPS on responses to Ureaplasma species have not been studied.
Our data have several clinical implications. Although Ureaplasma spp. are the organisms most commonly associated with chorioamnionitis, recent studies have confirmed the polymicrobial flora in many cases of early gestation chorioamnionitis 4, 30. Our findings suggest the possibility of decreased fetal inflammation in response to Gram-negative organisms with concomitant ureaplasma exposure. Since the timing of ureaplasma infections is not known in clinical cases, our findings of endotoxin tolerance with acute but not chronic ureaplasma infection also underline the complexity of interactions and difficulty in predicting severity of chorioamnionitis in women with mixed flora. Adults with sepsis have diminished innate immune responses 31. Postnatal nosocomial sepsis occurs in about 25% of very low birth weight preterm neonates 32. Possibly, ureaplasma chorioamnionitis could diminish innate immune responses and thereby increase the susceptibility of preterm infant to nosocomial sepsis.
Acknowledgments
This work was supported by National Institutes of Health Grants, HD 57869 (to S.G.K) and HL 97064 (to A.H.J. and S.G.K)
The authors would like to thank Clare Berry, PhD, Megan McAuliffe B.S., Manual Alvarez, Jr. M.S, Noah Hillman, M.D, Paranthaman Senthamarai Kannan PhD, and Amy Whitescarver B.S. for their expert technical assistance.
Footnotes
Disclosure: None of the authors have a conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- 2.Gravett MG, Rubens CE, Nunes TM. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth. 2010;10 (Suppl 1):S2. doi: 10.1186/1471-2393-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. American Journal of Obstetrics and Gynecology. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 4.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110, e111–117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37:393–409. doi: 10.1016/j.clp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 7.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 8.Dando SJ, Nitsos I, Kallapur SG, et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS One. 2012;7:e29856. doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallapur SG, Kramer BW, Knox CL, et al. Chronic Fetal Exposure to Ureaplasma parvum Suppresses Innate Immune Responses in Sheep. J Immunol. 2011;187:2688–2695. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 11.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 12.Kallapur SG, Kramer BW, Moss TJ, et al. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L633–L642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- 13.Dando SJ, Nitsos I, Newnham JP, Jobe AH, Moss TJ, Knox CL. Maternal administration of erythromycin fails to eradicate intrauterine ureaplasma infection in an ovine model. Biol Reprod. 2010;83:616–622. doi: 10.1095/biolreprod.110.084954. [DOI] [PubMed] [Google Scholar]
- 14.Knox CL, Dando SJ, Nitsos I, et al. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod. 2010;83:415–426. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 16.Berry CA, Nitsos I, Hillman NH, et al. Interleukin 1 in Lipopolysaccharide Induced Chorioamnionitis in the Fetal Sheep. Reprod Sci. 2011 doi: 10.1177/1933719111404609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallapur SG, Moss TJ, Auten RL, Jr, et al. IL-8 signaling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. Am J Physiol Lung Cell Mol Physiol. 2009;297:L512–519. doi: 10.1152/ajplung.00105.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah TA, Hillman NH, Nitsos I, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68:210–215. doi: 10.1203/PDR.0b013e3181e9c556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiology. 2008;154:1318–1325. doi: 10.1099/mic.0.2007/016212-0. [DOI] [PubMed] [Google Scholar]
- 21.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Paralanov V, Lu J, Duffy LB, et al. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012;12:88. doi: 10.1186/1471-2180-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 24.Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007;166:786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- 25.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 28.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67:117–121. doi: 10.1016/j.diagmicrobio.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 30.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]