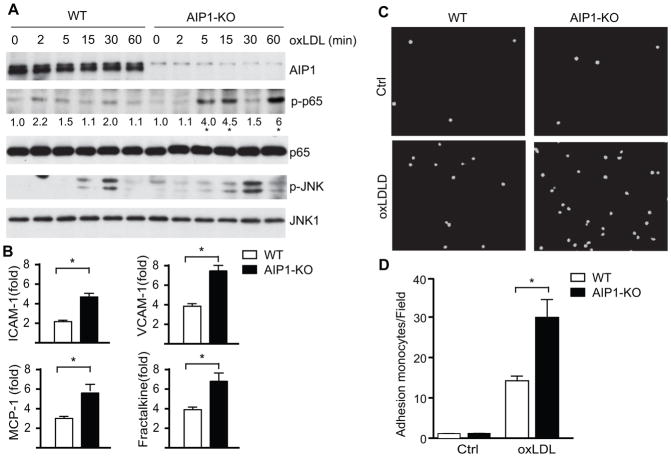
Figure 7. AIP1 deletion in EC significantly enhances oxLDL-induced NF-κB signaling in EC, but not in macrophages.
1×106 of WT (C57BL/6) and AIP1-KO mouse aortic EC were treated with 100 μg/ml oxLDL for the indicated times. Phospho- and total p65 and p-JNK1/2 were determined by immunoblotting with the respective antibodies. Total JNK1, AIP1 and β-actin were also determined. Representative blots from three independent experiments are shown. The quantification of the ratios of p-p65/p65 are presented, with untreated WT as 1.0. *, p<0.05 comparing WT vs AIP1-KO. B. WT and AIP1-KO mouse aortic EC were treated with 100 μg/ml oxLDL for 12 h. Transcripts for EC adhesion molecules (ICAM-1 and VCAM-1) and macrophage recruitment chemokines (MCP-1 and fractalkine) were quantified by qRT-PCR and normalized to hypoxanthine guanine phosphoribosyltransferase (HPRT). oxLDL-induced gene expression (fold of increase) are presented. Data are mean±SEM from three independent experiments. *, p<0.05. C–D. AIP1 deletion enhances leukocyte-endothelial adhesion. Isolated WT and AIP1-KO mouse aortic EC were seeded in 6 well plate (5×106 cells/well) as confluent monolayer for 24 h, followed by treatment with 100 μg/ml oxLDL for the other 24 h. Afterwards, RAW 264.7 cells, a mouse leukaemic monocyte macrophage cell line, were pre-stained with live cell tracker CMFDA and loaded onto mouse aortic EC layer (1×106 cells/well) for 1 h. Non-adherent cells were then removed by washing with PBS and adherent cells underwent immunofluorescence microscopy, with quantification of fluorescein isothiocyanate (FITC) units. Representative images are shown in panel C, with quantifications in panel D. Data are mean±SEM from three independent experiments. *, p<0.05.