Summary
Pregnancy following solid organ transplantation, although considered high risk for maternal, fetal and neonatal complications, has been quite successful. Tacrolimus pharmacokinetic changes during pregnancy make interpretation of whole blood trough concentrations particularly challenging. There are multiple factors that can increase the fraction of unbound tacrolimus, including but not limited to low albumin concentration and low RBC count. The clinical titration of dosage to maintain whole blood tacrolimus trough concentrations in the usual therapeutic range can lead to elevated unbound concentrations and possibly toxicity in pregnant women with anemia and hypoalbuminemia. Measurement of plasma or unbound tacrolimus concentrations for pregnant women might better reflect the active form of the drug, though these are technically-challenging and often unavailable in usual clinical practice. Tacrolimus crosses the placenta with in utero exposure being approximately 71% of maternal blood concentrations. The lower fetal blood concentrations are likely due to active efflux transport of tacrolimus from the fetus toward the mother by placental P-glycoprotein. To date, tacrolimus has not been linked to congenital malformations, but can cause reversible nephrotoxicity and hyperkalemia in the newborn. In contrast, very small amounts of tacrolimus are excreted in the breast milk and are unlikely to elicit adverse effects in the nursing infant.
Keywords: tacrolimus, pregnancy, nephrotoxicity, pharmacokinetics, protein binding
There is a growing body of evidence describing successful pregnancies in solid organ transplant recipients with tacrolimus based immunosuppression (1–5). Nevertheless, these pregnancies are at increased risk for maternal complications such as preeclampsia, hypertension, renal impairment, rejection, infection, post-pregnancy graft loss and miscarriage. Fetal and neonatal complications include preterm birth, stillbirth, intrauterine growth restriction, low birth weight, reversible renal dysfunction as well as hyperkalemia in the newborn and rarely neonatal death (1–5). Given the severity of the complications, a critical evaluation and optimization of tacrolimus therapy during pregnancy is warranted.
Immune Response
Immune changes in pregnancy are complex and are not detailed here in their entirety. A recent review of this topic has been published by Pazos et al. and can be refered to for a more detailed discussion (6). In brief, pregnancy creates a unique immune environment in which the fetus can be tolerated by down regulation of some T-cell mediated immune responses, while other components of the immune system such as monocytes and neutrophils are activated (7, 8). T cells, B cells, neutrophils, dendritic cells, monocytes and natural killer cells are transcriptionally regulated by estrogen (9, 10). CD3, CD4 and CD8 T cell concentrations in blood are decreased during pregnancy, but the CD4:CD8 ratio is maintained (8). The effects of pregnancy on cytokines are mixed. Some report increased concentrations of IL-2, IL-6 and IL-8 while others describe down regulation of INFγ, TNFα, IL-6, IL-10 and IL-13 but no change in IL-4 (8, 11–15). Pregnancy appears to promote a generalized activation of circulating leukocytes with up-regulation of adhesion molecules. B cell lymphopoiesis and total B cell counts are reduced (8, 16); however, estrogen has been shown to increase antibody production in vitro and in animal models (17, 18). Thus, it is possible that pregnancy decreases production of new B cells while at the same time enhancing antibody production from mature B cells (8). Although there is good evidence to suggest that some aspects of the immune response are dampened during pregnancy leading to decreased viral clearance and alleviation of symptoms for some autoimmune diseases, other parts of innate immunity are enhanced (6, 8).
There is a general aversion to using medications during pregnancy; however, discontinuation of immunosuppression during pregnancy has led to rejection and at least 2 maternal deaths, suggesting that some level of immunosuppression is required (16, 17). Published data with a variety of immunosuppressives, given alone or in combination, with or without dosage escalation during pregnancy suggest similar rejection rates as in the non-pregnant transplant population (19, 20). Rejection rates during pregnancy range from 2–8% (21, 22). Post-pregnancy graft loss at 2 years is approximately 8% (22). The effect of pregnancy on allograft function remains controversial (22, 23).
Physiologic Changes in Pregnancy that Alter Drug Disposition
There are a number of physiologic changes that occur during pregnancy that alter biotransformation, renal clearance, volume of distribution and protein binding. Pregnancy increases the activities of some drug-metabolizing enzymes (e.g. CYP3A, CYP2D6, CYP2C9 and UGT), whereas others (e.g. CYP1A2 and CYP2C19) are decreased (24–29). Previous work utilizing probe substrates suggests that intrinsic CYP3A activity increases by 25–100% during pregnancy (24, 26, 30, 31).
Not only are there changes in drug metabolism during pregnancy, but also in renal clearance (24, 32–34). Normal pregnancy is characterized by renal vasodilation. Effective renal plasma flow increases significantly by 6 weeks gestation, peaking ~50–85% above non-pregnant values, with a corresponding increase in glomerular filtration rate (GFR) (35–37). Normal serum creatinine during pregnancy is ≤ 0.7 mg/dL. In addition, there appears to be an increase in the activity of several renal transporters (e.g. P-glycoprotein) (24, 33, 34). Changes in enzyme and transporter activities, as well as renal filtration can increase or decrease concentrations of many drugs (24, 25, 32).
Average weight gain during pregnancy is ~13.5 kg for women with normal BMI (38). The majority of the weight gain during pregnancy is water (~62%), followed by fat (~30%) and protein (~8%). Along with the increase in total body water (~7–9 L) are parallel increases in extracellular fluid and blood volumes (36, 39). Changes in body composition along with those in protein binding can result in changes in the volume of distribution for some drugs. Although an increase in volume of distribution alone will not alter average steady state drug concentrations, it will lead to lower peaks and higher troughs.
Changes in drug binding in blood are most clinically relevant when the percentage bound is high (>80%). Drugs can bind to plasma proteins such as albumin and α1-acid glycoprotein (AAG) or to cellular components such as erythrocytes or lymphocytes. Albumin concentrations in normal pregnancy decrease by ~1–13% across gestation and by much more in some allograft recipients (29, 40). Pregnancy also leads to lower plasma AAG concentrations (~52% lower at 30–36 weeks gestation) (41). Hemoglobin is known to fall by ~11% during normal pregnancy (nadirs mid-pregnancy); however, much greater declines and anemia have been reported following solid organ transplantation (29, 40). Depending on the extraction ratio (ER) of the drug across the eliminating organ (liver, kidney), changes in drug binding may or may not affect drug clearance, volume of distribution and first-pass metabolism.
For most drugs, particularly those that have low ERs, a decrease in drug binding to plasma proteins can lead to lower total drug concentrations, but no change in unbound concentrations (active form of the drug) (29). A well-documented example of this is phenytoin. For phenytoin, low albumin concentrations result in lower total drug concentrations but no change in unbound concentrations (42). For this reason, clinicians either correct the total phenytoin concentrations for changes in binding or measure unbound concentrations and titrate to the unbound therapeutic range. Like phenytoin, a number of other agents that have been reported to have an increased fraction unbound during pregnancy (e.g. tacrolimus, lopinavir, valproic acid, phenobarbital, dexamethasone, and propranolol) (41, 43).
Tacrolimus Pharmacokinetics in Pregnancy
The complexity of tacrolimus pharmacokinetics makes it particularly interesting from the research perspective, but challenging from the clinical perspective. Tacrolimus is a substrate for CYP3A4, CYP3A5 and P-glycoprotein (44, 45). It is also highly bound to plasma proteins and erythrocytes. In addition, it has temperature and concentration dependent partitioning between plasma and red blood cells (RBCs). Using blood for analysis, tacrolimus is considered to be a low ER drug. However with plasma, it could be considered a high ER drug. These characteristics, along with the slow tacrolimus uptake and release by erythrocytes, make predicting the pharmacokinetic changes during pregnancy difficult.
Many case reports discuss the effects of pregnancy on tacrolimus concentrations. Some describe no change in tacrolimus concentrations or dosage while others report lower concentrations and / or the need for dosage escalation during pregnancy (1, 2, 4, 46–52). Interpretation of these reports has been limited by differences in the biologic fluid (blood, plasma, or serum), non-specific methodologies (ELISA, FPIA), lack of attention to temperature dependent distribution into RBCs and failure to account for changes in plasma proteins or RBC concentrations on drug binding. These issues have been addressed by Zheng et al. (29). This work highlights three important findings described below, which explain the discrepancies in previous reports. First, tacrolimus fraction unbound and whole blood oral clearance increase during pregnancy. Second, the changes in whole blood drug concentrations reflect changes in drug binding due to low RBC count, albumin and perhaps AAG concentrations during pregnancy. Third, there is no change in whole blood, unbound oral clearance. The following discussion will address the clinical implications of these findings in pregnant allograft recipients. We will also comment on tacrolimus in utero exposure and neonatal exposure through breast milk.
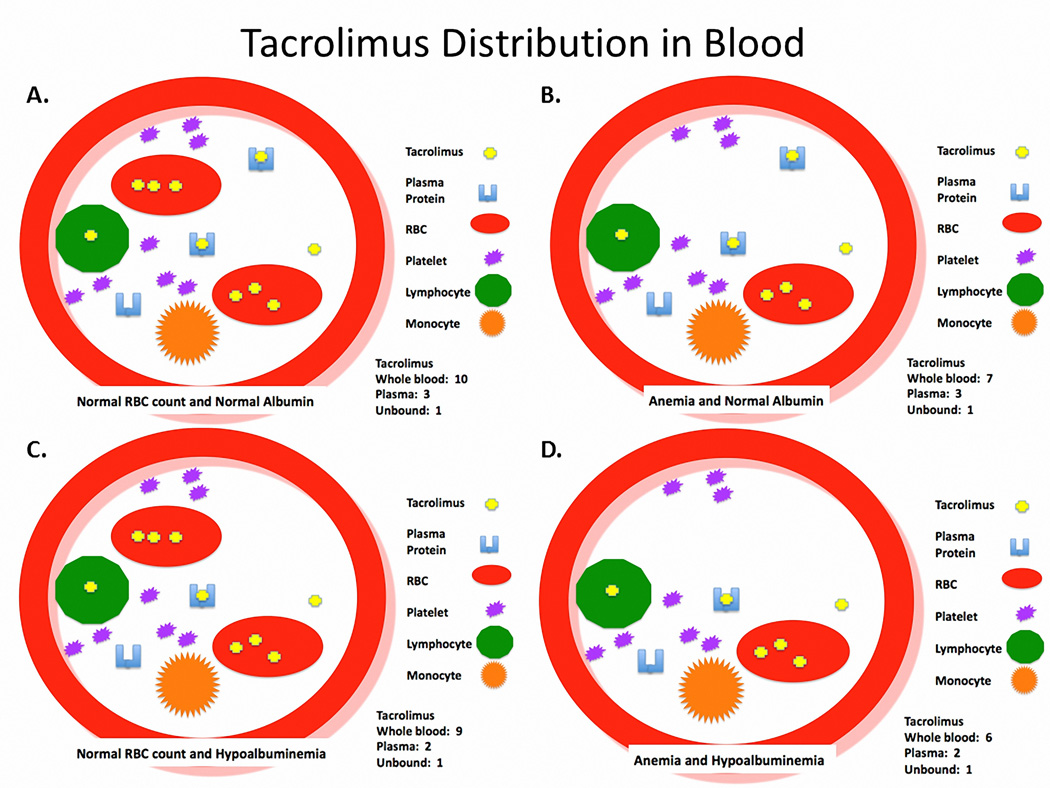
Tacrolimus Distribution in Blood
Tacrolimus concentrates in erythrocytes, with blood-to-plasma ratio ranging from 4:1 to 42:1 (29, 53). Tacrolimus binding to erythrocytes accounts for 85–95% of the drug in blood, which slowly equilibrates with plasma (54–56). Patients with higher RBC counts or lower tacrolimus concentrations exhibit greater tacrolimus blood-to-plasma ratios (29, 57). In pregnancy, RBC counts decrease as a result of rapid volume expansion and relatively greater increase in plasma volume than RBC mass (58). Tacrolimus is highly protein bound (< 3% unbound) to both albumin and AAG in plasma, accounting for 5–15% of the drug in blood (29, 55, 59). Changes in albumin, AAG and RBCs will alter tacrolimus binding (29, 60). Therefore, if there is no change in the unbound intrinsic clearance of tacrolimus, a decrease in albumin, AAG and / or RBC count should lead to a fall in whole blood concentration, but no change in unbound concentration. Figure 1A–D depict this concept by comparing the distribution profile within the blood in normal, anemic and / or hypoalbuminemic patients without any adjustment in dosage or change in intrinsic clearance of unbound drug. Figure 1A is a state of normal RBC count and albumin concentration. Tacrolimus is concentrated in the RBCs and most of the tacrolimus in plasma is bound to plasma proteins. In this example, the whole blood concentration is 10, plasma concentration is 3, and unbound concentration is 1. Figure 1B and 1C are examples of the same situation except the patient has anemia and hypoalbuminemia, respectively. The active form of the drug (unbound concentration) in both cases is still 1, but the whole blood concentrations are now 7 and 9, respectively. In Figure 1D the patient has anemia and hypoalbuminemia as is often seen during pregnancy in allograft recipients. In this example, the unbound concentration stayed the same at 1, but the whole blood concentration fell to 6. Conceptually, this is the same situation for tacrolimus during pregnancy in allograft recipients described by Zheng et al. (29).
Figure 1.
Depicts a theoretical representation of tacrolimus distribution in blood of patients with A. normal red blood cell count and normal albumin, B. anemia and normal albumin, C. normal red blood cell count and hypoalbuminemia and D. anemia and hypoalbuminemia.
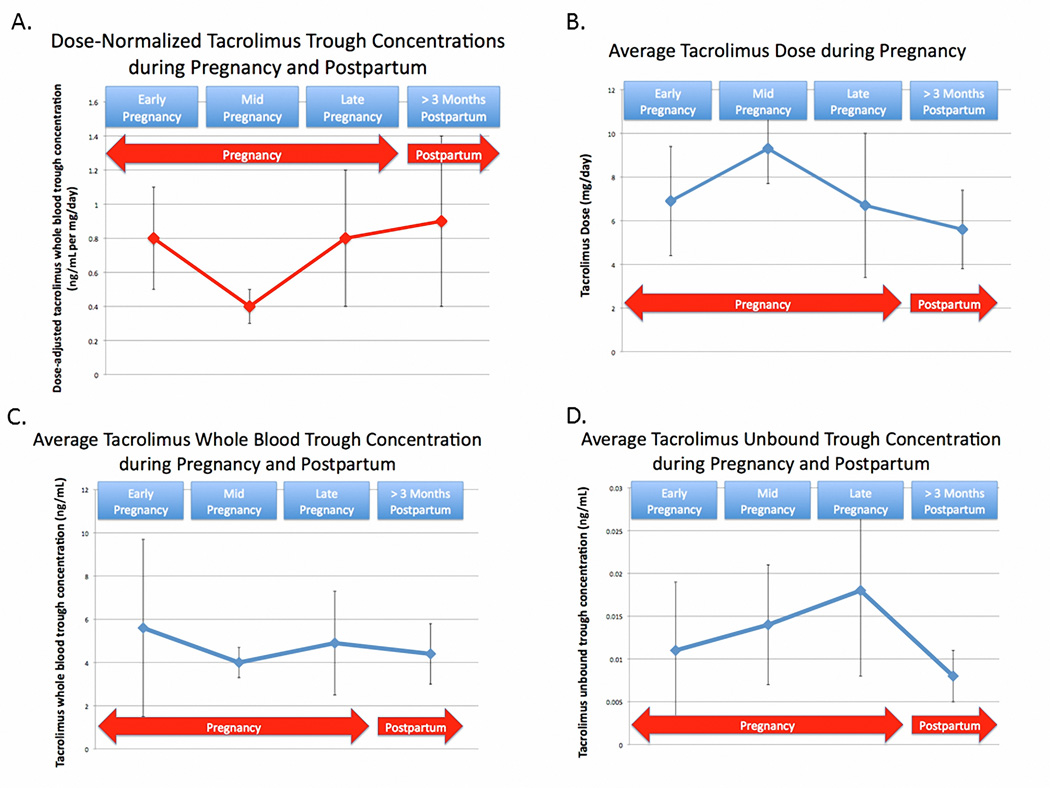
Clinically, trough tacrolimus concentrations are measured in whole blood, thus dosage titration to maintain trough concentrations in the therapeutic range leads to an increase in unbound tacrolimus concentrations. With this increase comes the potential for tacrolimus toxicity. To illustrate the clinical implications of this, the subjects participating in the Zheng et al. study are presented in Figure 2A–D and Table 1. The whole blood oral clearance for tacrolimus is 39% higher during pregnancy compared to postpartum. The consequence of the increase in oral clearance in pregnancy can be graphically seen in Figure 2A, in which the dose-normalized, whole blood, trough concentrations are lower during pregnancy than postpartum. If no dosage adjustments had been made, the whole blood trough concentrations would have fallen. However, as this started to occur, the clinicians increased the tacrolimus dosage (Figure 2B). In doing so, they were quite successful at maintaining whole blood trough concentrations within the target range as seen in Figure 2C. However, because the tacrolimus whole blood unbound fraction increased by ~100% during pregnancy and there was no change in tacrolimus unbound oral clearance in blood, without realizing it, these dosage adjustments resulted in a markedly higher unbound tacrolimus trough concentrations (active form), as shown in Figure 2D. Although maintaining whole blood tacrolimus trough concentrations in the usual therapeutic range during pregnancy has been routine in many transplant centers, it is unclear if this is the optimum approach.
Figure 2.
A. Average (± SD), dose-normalized (1 mg/day), whole blood tacrolimus trough concentrations during early-, mid-, and late-pregnancy as well as > 3 months postpartum. B. Average (± SD) tacrolimus dose (mg/day) during early-, mid-, and late-pregnancy as well as > 3 months postpartum. C. Average (± SD) whole blood tacrolimus trough concentrations during early-, mid-, and late-pregnancy as well as > 3 months postpartum. D. Average (± SD) unbound tacrolimus trough concentrations during early-, mid-, and late-pregnancy as well as > 3 months postpartum.
Table 1.
Average tacrolimus dose, whole blood and unbound trough concentrations during early-, mid-,and late-pregnancy as well as > 3 months postpartum
| Early- pregnancy |
Mid- pregnancy |
Late- pregnancy |
>3 Month Postpartum |
|
|---|---|---|---|---|
| Dose-Norm alized (per 1 mg/day) Tacrolim us Trough Concentrations (ng/m L) |
0.8 ± 0.03 | 0.4 ± 0.1 | 0.8 ± 0.04 | 0.9 ± 0.5 |
| Average Tacrolim us Dose (mg) | 6.9 ± 2.5 | 9.3 ± 1.6 | 6.7 ± 3.3 | 5.6 ± 1.8 |
| Average Tacrolim us Whole Blood Trough Concentration (ng/mL) |
5.6 ± 4.1 | 4.0 ± 0.7 | 4.9 ± 2.4 | 4.4 ± 1.4 |
| Average Tacrolimus Unbound Trough Concentration (ng/m L) |
0.011 ± 0.008 |
0.014 ± 0.007 |
0.018 ± 0.010 |
0.008 ± 0.003 |
Establishing the optimum therapeutic range for tacrolimus during pregnancy is not possible based on the paucity of available data. The impacts of tacrolimus unbound and plasma concentrations have been evaluated for efficacy in the non-pregnant population. The concentration of tacrolimus in the blood primarily reflects RBC concentration, which does not necessarily reflect lymphocyte concentration or its availability to interact with intracellular targets (61, 62). Several experts suggest that unbound concentration of tacrolimus better correlates with incidence of rejection (63–65). Unbound drug, which is available for cellular diffusion and cellular distribution, may be more reflective of target site concentration. Indeed, tacrolimus plasma concentrations in patients are in the range of in vitro lymphocyte proliferation inhibitory concentrations (66–68). Zahir et al. reported that the percentage of tacrolimus associated with the lymphocytes and unbound concentration in blood were significantly higher in stable allograft recipients than in those experiencing rejection (69). Tsunoda et al. suggested that concentrations in the transplanted organ itself might be more predictive of the pharmacological effect of tacrolimus (70). Hepatic tissue concentrations of tacrolimus were found to be significantly higher in patients without rejection than in patients experiencing rejection episodes after liver transplantation (71). Based on the data from Zheng et al., (29), it is expected that if the tacrolimus dose were not increased during pregnancy to maintain a supposed therapeutic whole blood trough concentrations, the unbound tacrolimus concentration in blood would be comparable during pregnancy and postpartum. This assumes that no other factors alter the unbound tacrolimus concentrations. Given the high variability in tacrolimus pharmacokinetics, this assumption will not always hold. Even so, in many patients, the therapeutic benefit of tacrolimus may not be compromised by withholding dosage adjustment unless warranted by other confounding variables. Consistent with this, Jain et al. reported no rejection episodes in 21 pregnancies in which tacrolimus dosage was not adjusted during gestation despite lower trough concentrations (2).
Tacrolimus Therapeutic Range in Pregnancy
Only whole blood concentrations are currently available in the clinical setting, thus careful consideration should be given to those patients that have significant anemia and hypoalbuminemia. Multiple studies have reported correlations between RBC count and whole blood tacrolimus concentrations (29, 60, 72). However, the unbound concentration should not change if no dosage adjustments are made and no other factors occur that would alter tacrolimus concentrations. Due to the variability in tacrolimus concentrations, the challenges with treating rejection during pregnancy and the potential for tacrolimus whole blood concentrations to fall below the lower limits of assay capability, clinicians are likely to be concerned about not adjusting the tacrolimus dosage. If the decision is made to maintain whole blood trough concentrations in the usual therapeutic range during pregnancy in hypoalbuminemic and/or anemic patients, it must be recognized that unbound concentrations might be double those measured prior to pregnancy. The choice of target whole blood concentrations should take into account the individual patient’s history, current medical conditions, concomitant medications and the potential impact of high or low unbound concentrations.
Toxicity
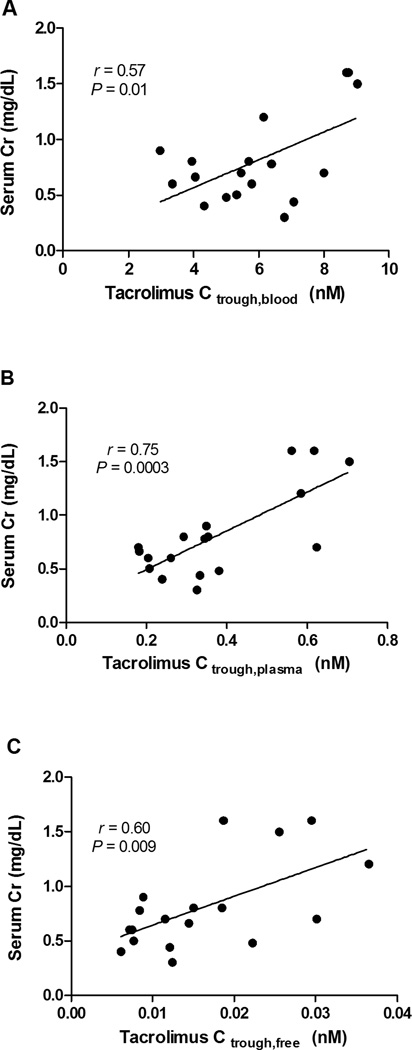
Not only is it important to consider the effects of tacrolimus concentrations on efficacy, but toxicity as well. Trull et al. (73) reported that in non-pregnant liver allograft recipients receiving tacrolimus who were randomized post-transplantation to 2 weeks of albumin infusion or artificial plasma expander, those with the albumin infusions and corresponding higher serum albumin concentrations had lower serum creatinine concentrations and higher rejection rates. No significant differences were observed in whole blood tacrolimus concentrations between groups. It was suggested that the greater unbound fraction of tacrolimus in the artificial plasma expander group might have led to an increase in nephrotoxicity and lower rejection rate by increasing the active form of the drug. Excluding the patient experiencing rejection during pregnancy from the subjects studied by Zheng et al. (29), Figure 3A–C demonstrates an apparent correlation between serum creatinine concentration and tacrolimus trough whole blood, plasma and unbound blood concentrations, respectively. Although the data are limited, tacrolimus nephrotoxicity during pregnancy appears to correlate best with trough plasma concentrations. In normal pregnancies, a substantial increase is expected in creatinine clearance. Not surprisingly, given the doubling of unbound tacrolimus concentrations observed by Zheng et al. (29), the expected increase in creatinine clearance during mid-/late-pregnancy (115.2 ± 54.3 mL/min) as compared to postpartum (129.8 ± 52.2 mL/min) was not observed. Accordingly, the attenuated creatinine clearance during pregnancy may reflect tacrolimus nephrotoxicity. Backman et al. (74) suggested that monitoring tacrolimus plasma concentrations would be superior to whole blood, based the reduction in GFR correlating with yearly mean tacrolimus plasma but not with whole blood concentrations or dosage. Notably, Zheng et al. (29) observed a strong correlation between tacrolimus plasma and unbound concentrations in blood (r = 0.9, P < 0.0001), whereas a weaker correlation existed between whole blood and unbound concentrations (r = 0.6, P = 0.004).
Figure 3.
Correlations between serum creatinine concentrations during pregnancy and postpartum and A. blood, B. plasma and C. unbound tacrolimus trough concentrations in non-rejecting transplantation recipients
In addition to nephrotoxicity, other tacrolimus side effects are likely to be worsened due to the elevated unbound concentrations during pregnancy. For example, pregnancy complications in kidney transplant recipients include high rates of infection (22%), hypertension (56%), preeclampsia (32%) and low birth-weight infants (46%) (5). In addition, diabetes occurs in 8% of the kidney transplant patients receiving tacrolimus during pregnancy. We were unable to locate published reports on the frequency of tacrolimus-induced neurotoxicity during pregnancy. For all of these adverse effects, other than nephrotoxicity, data are lacking on concentration-response during pregnancy to evaluate the effects of elevated unbound tacrolimus concentrations on their incidence and severity.
Fetal Exposure
Tacrolimus, like most drugs, crosses the placenta, with umbilical cord concentrations being ~71%, 23% and 19% of maternal concentrations for whole blood, plasma and unbound, respectively. (75). The downward concentration gradient from maternal circulation to umbilical cord probably reflects the active efflux of tacrolimus from the fetus toward the mother by placental P-glycoprotein as well as the difference between maternal and fetal hematocrits. Even with some transfer across the placenta, tacrolimus does not appear to cause congenital malformations (1). However, tacrolimus has been reported to cause reversible neonatal hyperkalemia and renal impairment (1). In addition, intrauterine growth restriction as well as premature delivery due to hypertension, preeclampsia and premature rupture of membranes have been reported (1, 47, 76, 77). The long-term effects of in utero exposure to tacrolimus are not known, but the usual well-known complications from prematurity and/or low birth weight are expected to be observed in the infant when part of the clinical picture. Further research is needed to evaluate the long-term effects of in utero exposure to tacrolimus on outcomes in the offspring including neurobehavioral, cardiovascular, renal, endocrine, immunologic and oncologic.
Breast Milk
Tacrolimus is excreted in the breast milk with infant ingestion reported to be < 1% of the maternal weight-adjusted dosage (75, 78, 79). Breastfeeding does not appear to contribute to tacrolimus concentrations in the infant postpartum (80). A limited number of cases report no adverse effects in the nursing infants while the mother was receiving tacrolimus therapy (79–81). The American Academy of Pediatrics has listed cyclosporine in their table entitled “Cytotoxic drugs may interfere with cellular metabolism of the nursing infant” (82). Theoretically, calcineurin inhibitors might alter the immune benefits transferred to the nursing infant, however no reports were found. Similar to most medications, product labeling for tacrolimus states that nursing should be avoided. However, the amount that is excreted through the breast milk is extremely low and unlikely to have any effect on the nursing infant. Infant exposure during lactation is expected to be far lower than in utero exposure. Blood level monitoring of the infant while being mindful of the effects of plasma proteins and RBC count on drug binding could be considered if concerns arose.
Conclusions
For pregnant transplant recipients with anemia and/or hypoalbuminimia, monitoring plasma or unbound tacrolimus trough concentrations, although more costly and less readily accessible, might better predict drug efficacy and toxicity than whole blood concentrations (29, 32, 67, 74). Significant changes occur in tacrolimus whole blood oral clearance when the RBC count falls below 3.5 million/μL and in the percent unbound when albumin concentration falls below 3.0 G/dL in pregnant transplant recipients. Therefore, if tacrolimus whole blood concentrations are the only available assay, clinical interpretation of trough tacrolimus concentrations for these women should take into account RBC count and serum albumin concentration. A couple of proposed strategies are available to clinicians caring for pregnant transplant recipients with hypoalbuminemia and/or anemia. 1. Maintain whole blood concentrations in the usual therapeutic range, until she develops signs and symptoms of tacrolimus toxicity then decrease the dose, or 2. Do not adjust the dose unless the concentrations fall by more than 50% or they fall below the lower limit of the clinical assay, then increase the dose. This assumes that no other factors exist that lower tacrolimus concentrations. The first approach will increase the patient’s risk for toxicity and the second for rejection. Following in utero exposure to tacrolimus, no evidence of congenital malformations has been identified. Tacrolimus appears to be compatible with breastfeeding.
Acknowledgments
Research support:
The project described was supported by grants numbers U10HD047892, U10HD047891 and U10HD047890 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institute of Health / National Center for Research Resources grants UL1RR025014, and UL1RR031975 and National Institutes of Health R01 GM068871. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Abbreviations
- AAG
α1-acid glycoprotein
- B cell
B lymphocyte
- CD3
cluster of differentiation 3 cells
- CD4
cluster of differentiation 4 cells
- CD8
cluster of differentiation 8 cells
- CYP1A2
cytochrome P450 1A2
- CYP2C9
cytochrome P450 2C9
- CYP2C19
cytochrome P450 2C19
- CYP2D6
cytochrome P450 2D6
- CYP3A4
cytochrome P450 3A4
- CYP3A5
cytochrome P450 3A5
- ELISA
enzyme-linked immunosorbent assay
- ER
extraction ratio, the relative efficiency of eliminating the drug from the systemic circulation on a single pass through the organ
- FPIA
fluorescence polarization immunoassay
- GFR
glomerular filtration rate
- L
liter
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-6
interleukin 6
- IL-8
interleukin 8
- IL-10
interleukin 10
- IL-13
interleukin 13
- INF
interferon gamma
- OAT
organic anion transporter
- OCT
organic cation transporter
- RBC
red blood cell
- SD
standard deviation
- T cell
T lymphocyte
- TNF
tumor necrosis factor alpha
- UGT
uridine 5'-diphospho-glucuronosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
Mary F. Hebert, Participated in research design, Participated in writing the paper, Participated in performance of the research, Participated in data analysis
Songmao Zheng, Participated in writing the paper, Participated in performance of the research, Participated in data analysis
Karen Hays, Participated in performance of the research, Participated in writing the paper
Danny D. Shen, Participated in writing the paper, Participated in data analysis
Connie L. Davis, Participated in writing the paper
Jason Umans, Participated in research design, Participated in performance of the research, Participated in writing the paper
Menachem Miodovnik, Participated in research design, Participated in performance of the research, Participated in writing the paper
Kenneth Thummel, Participated in writing the paper, Participated in performance of the research, Participated in data analysis
Thomas R. Easterling, Participated in research design, Participated in writing the paper, Participated in performance of the research
Conflicts of interest:
No conflict of interest
References
- 1.Kainz A, Harabacz I, Cowlrick IS, Gadgil SD, Hagiwara D. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70(12):1718. doi: 10.1097/00007890-200012270-00010. [DOI] [PubMed] [Google Scholar]
- 2.Jain AB, Shapiro R, Scantlebury VP, et al. Pregnancy after kidney and kidney-pancreas transplantation under tacrolimus: a single center's experience. Transplantation. 2004;77(6):897. doi: 10.1097/01.tp.0000117564.50117.fb. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez MJ, Acebedo-Ribo M, Garcia-Donaire JA, et al. Pregnancy in renal transplant recipients. Transplant Proc. 2005;37(9):3721. doi: 10.1016/j.transproceed.2005.09.175. [DOI] [PubMed] [Google Scholar]
- 4.Jain AB, Reyes J, Marcos A, et al. Pregnancy after liver transplantation with tacrolimus immunosuppression: a single center's experience update at 13 years. Transplantation. 2003;76(5):827. doi: 10.1097/01.TP.0000084823.89528.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2010:65. [PubMed] [Google Scholar]
- 6.Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res. 2012 doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 8.Kraus TA, Engel SM, Sperling RS, et al. Characterizing the Pregnancy Immune Phenotype: Results of the Viral Immunity and Pregnancy (VIP) Study. Journal of Clinical Immunology. 2012;32(2):300. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180(1):630. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 10.Pierdominici M, Maselli A, Colasanti T, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132(1–2):79. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Luppi P, Haluszczak C, Betters D, Richard CAH, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. Journal of Leukocyte Biology. 2002;72(5):874. [PubMed] [Google Scholar]
- 12.Luppi P, Haluszczak C, Trucco M, Deloia JA. Normal pregnancy is associated with peripheral leukocyte activation. American Journal of Reproductive Immunology. 2002;47(2):72. doi: 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- 13.Wegmann TG. The Cytokine Basis for Cross-Talk between the Maternal Immune and Reproductive Systems. Current Opinion in Immunology. 1990;2(5):765. doi: 10.1016/0952-7915(90)90048-l. [DOI] [PubMed] [Google Scholar]
- 14.Austgulen R, Lien E, Liabakk NB, Jacobsen G, Arntzen KJ. Increased Levels of Cytokines and Cytokine Activity Modifiers in Normal-Pregnancy. European Journal of Obstetrics Gynecology and Reproductive Biology. 1994;57(3):149. doi: 10.1016/0028-2243(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 15.Opsjon SL, Wathen NC, Tingulstad S, et al. Tumor-Necrosis-Factor, Interleukin-1, and Interleukin-6 in Normal Human-Pregnancy. American Journal of Obstetrics and Gynecology. 1993;169(2):397. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 16.Medina KL, Smithson G, Kincade PW. Suppression of B-Lymphopoiesis during Normal-Pregnancy. Journal of Experimental Medicine. 1993;178(5):1507. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. Journal of Allergy and Clinical Immunology. 1999;103(2):282. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 18.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: Expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. Journal of Immunology. 2001;167(4):1886. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 19.McKay DB, Josephson MA. Pregnancy in recipients of solid organs--effects on mother and child. N Engl J Med. 2006;354(12):1281. doi: 10.1056/NEJMra050431. [DOI] [PubMed] [Google Scholar]
- 20.Sims CJ. Organ transplantation and immunosuppressive drugs in pregnancy. Clin Obstet Gynecol. 1991;34(1):100. doi: 10.1097/00003081-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Armenti VT, Daller JA, Constantinescu S, et al. Report from the National Transplantation Pregnancy Registry: outcomes of pregnancy after transplantation. Clin Transpl. 2006:57. [PubMed] [Google Scholar]
- 22.Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11(11):2388. doi: 10.1111/j.1600-6143.2011.03656.x. [DOI] [PubMed] [Google Scholar]
- 23.Galdo T, Gonzalez F, Espinoza M, et al. Impact of pregnancy on the function of transplanted kidneys. Transplant Proc. 2005;37(3):1577. doi: 10.1016/j.transproceed.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Hebert MF, Easterling TR, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 25.Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85(6):607. doi: 10.1038/clpt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Claessens AJ, Risler LJ, Eyal S, Shen DD, Easterling TR, Hebert MF. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010;38(9):1393. doi: 10.1124/dmd.110.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan ML, Easterling TR, Carr DB, et al. Clonidine pharmacokinetics in pregnancy. Drug Metab Dispos. 2009;37(4):702. doi: 10.1124/dmd.108.024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng S, Easterling TR, Umans JG, et al. Pharmacokinetics of Tacrolimus During Pregnancy. Ther Drug Monit. 2012 doi: 10.1097/FTD.0b013e3182708edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirt D, Treluyer JM, Jullien V, et al. Pregnancy-related effects on nelfinavir-M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50(6):2079. doi: 10.1128/AAC.01596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62(3):309. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45(1):25. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 33.Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrew MA, Easterling TR, Carr DB, et al. Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther. 2007;81(4):547. doi: 10.1038/sj.clpt.6100126. [DOI] [PubMed] [Google Scholar]
- 35.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 36.Davison JM, Dunlop W. Renal Hemodynamics and Tubular Function in Normal Human-Pregnancy. Kidney Int. 1980;18(2):152. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 37.Sturgiss SN, Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8(2):209. doi: 10.1016/s0950-3552(05)80319-0. [DOI] [PubMed] [Google Scholar]
- 38.Buschur E, Kim C. Guidelines and interventions for obesity during pregnancy. Int J Gynaecol Obstet. 2012 doi: 10.1016/j.ijgo.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hytten FE, Thomson AM, Taggart N. Total body water in normal pregnancy. J Obstet Gynaecol Br Commonw. 1966;73(4):553. doi: 10.1111/j.1471-0528.1966.tb15533.x. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Washington DC: National Academy Press; 1990. Nutrition during pregnancy; p. 468. [Google Scholar]
- 41.Aweeka FT, Stek A, Best BM, et al. Lopinavir protein binding in HIV-1-infected pregnant women. Hiv Medicine. 2010;11(4):232. doi: 10.1111/j.1468-1293.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ME W. Basic Clinical Pharmacokinetics, Fourth Edition. . Troy DB. Baltimore: Lippincott Williams & Wilkins; 2004. p. 511. [Google Scholar]
- 43.Perucca E, Crema A. Plasma protein binding of drugs in pregnancy. Clin Pharmacokinet. 1982;7(4):336. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20(5):753. [PubMed] [Google Scholar]
- 45.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268(9):6077. [PubMed] [Google Scholar]
- 46.Grimer M. The CARI guidelines. Calcineurin inhibitors in renal transplantation: pregnancy, lactation and calcineurin inhibitors. Nephrology (Carlton) 2007;12(Suppl 1):S98. doi: 10.1111/j.1440-1797.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 47.Jain A, Venkataramanan R, Fung JJ, et al. Pregnancy after liver transplantation under tacrolimus. Transplantation. 1997;64(4):559. doi: 10.1097/00007890-199708270-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimura N, Oka T, Fujiwara Y, Ohmori Y, Yasumura T, Honjo H. A case report of pregnancy in renal transplant recipient treated with FK506 (tacrolimus) Transplantation. 1996;61(10):1552. doi: 10.1097/00007890-199605270-00025. [DOI] [PubMed] [Google Scholar]
- 49.Casele HL, Laifer SA. Association of pregnancy complications and choice of immunosuppressant in liver transplant patients. Transplantation. 1998;65(4):581. doi: 10.1097/00007890-199802270-00023. [DOI] [PubMed] [Google Scholar]
- 50.Midtvedt K, Hartmann A, Brekke IB, Lyngdal PT, Bentdal O, Haugen G. Successful pregnancies in a combined pancreas and renal allograft recipient and in a renal graft recipient on tacrolimus treatment. Nephrol Dial Transplant. 1997;12(12):2764. doi: 10.1093/ndt/12.12.2764. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Donaire JA, Acevedo M, Gutierrez MJ, et al. Tacrolimus as basic immunosuppression in pregnancy after renal transplantation. A single-center experience. Transplant Proc. 2005;37(9):3754. doi: 10.1016/j.transproceed.2005.09.124. [DOI] [PubMed] [Google Scholar]
- 52.Jabiry-Zieniewicz Z, Kaminski P, Pietrzak B, et al. Outcome of four high-risk pregnancies in female liver transplant recipients on tacrolimus immunosuppression. Transplant Proc. 2006;38(1):255. doi: 10.1016/j.transproceed.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 53.Sam WJ, Tham LS, Holmes MJ, et al. Population pharmacokinetics of tacrolimus in whole blood and plasma in asian liver transplant patients. Clin Pharmacokinet. 2006;45(1):59. doi: 10.2165/00003088-200645010-00004. [DOI] [PubMed] [Google Scholar]
- 54.Piekoszewski W, Chow FS, Jusko WJ. Disposition of tacrolimus (FK 506) in rabbits. Role of red blood cell binding in hepatic clearance. Drug Metab Dispos. 1993;21(4):690. [PubMed] [Google Scholar]
- 55.Chow FS, Piekoszewski W, Jusko WJ. Effect of hematocrit and albumin concentration on hepatic clearance of tacrolimus (FK506) during rabbit liver perfusion. Drug Metab Dispos. 1997;25(5):610. [PubMed] [Google Scholar]
- 56.Beysens AJ, Wijnen RM, Beuman GH, van der Heyden J, Kootstra G, van As H. FK 506: monitoring in plasma or in whole blood? Transplant Proc. 1991;23(6):2745. [PubMed] [Google Scholar]
- 57.Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther. 1995;57(3):281. doi: 10.1016/0009-9236(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 58.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14(3):601. [PubMed] [Google Scholar]
- 59.Undre NA. Pharmacokinetics of tacrolimus-based combination therapies. Nephrol Dial Transplant. 2003;18(Suppl 1):i12. doi: 10.1093/ndt/gfg1029. [DOI] [PubMed] [Google Scholar]
- 60.Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. European Tacrolimus Multicentre Renal Study Group. Transplant Proc. 1998;30(4):1261. doi: 10.1016/s0041-1345(98)00234-6. [DOI] [PubMed] [Google Scholar]
- 61.Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41(11):813. doi: 10.2165/00003088-200241110-00003. [DOI] [PubMed] [Google Scholar]
- 62.Ichimaru N, Takahara S, Kokado Y, et al. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis. 2001;158(2):417. doi: 10.1016/s0021-9150(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 63.Erden E, Warty V, Magnone M, Shapiro R, Demetris J, Randhawa P. Plasma FK506 levels in patients with histopathologically documented renal allograft rejection. Transplantation. 1994;58(3):397. [PubMed] [Google Scholar]
- 64.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62(7):920. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 65.Morozumi K. Morphological-Characteristics of Renal-Allografts Showing Renal Dysfunction under Fk-506 Therapy - Is Graft Biopsy Available to Reveal the Morphological Findings Corresponding with Fk-506 Nephropathy. Transplant Proc. 1993;25(1):624. [PubMed] [Google Scholar]
- 66.Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci. 1993;82(3):340. doi: 10.1002/jps.2600820325. [DOI] [PubMed] [Google Scholar]
- 67.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139(6):1797. [PubMed] [Google Scholar]
- 68.Eiras G, Imventarza O, Murase N, et al. Species differences in sensitivity of T lymphocytes to immunosuppressive effects of FK 506. Transplantation. 1990;49(6):1170. doi: 10.1097/00007890-199006000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57(3):298. doi: 10.1046/j.1365-2125.2003.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsunoda SM, Aweeka FT. The use of therapeutic drug monitoring to optimise immunosuppressive therapy. Clin Pharmacokinet. 1996;30(2):107. doi: 10.2165/00003088-199630020-00003. [DOI] [PubMed] [Google Scholar]
- 71.Sandborn WJ, Lawson GM, Cody TJ, et al. Early cellular rejection after orthotopic liver transplantation correlates with low concentrations of FK506 in hepatic tissue. Hepatology. 1995;21(1):70. [PubMed] [Google Scholar]
- 72.Minematsu T, Sugiyama E, Kusama M, et al. Effect of hematocrit on pharmacokinetics of tacrolimus in adult living donor liver transplant recipients. Transplant Proc. 2004;36(5):1506. doi: 10.1016/j.transproceed.2004.04.097. [DOI] [PubMed] [Google Scholar]
- 73.Trull A, Hughes V, Cooper D, et al. Influence of albumin supplementation on tacrolimus and cyclosporine therapy early after liver transplantation. Liver Transpl. 2002;8(3):224. doi: 10.1053/jlts.2002.31347. [DOI] [PubMed] [Google Scholar]
- 74.Backman L, Nicar M, Levy M, et al. FK506 trough levels in whole blood and plasma in liver transplant recipients. Correlation with clinical events and side effects. Transplantation. 1994;57(4):519. [PubMed] [Google Scholar]
- 75.Zheng S, Easterling Thomas R, Hays Karen, et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk (submitted to Pediatrics) 2012 doi: 10.1111/bcp.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain A, Venkataramanan R, Lever J, et al. FK506 and pregnancy in liver transplant patients. Transplantation. 1993;56(6):1588. [PMC free article] [PubMed] [Google Scholar]
- 77.Resch B, Mache CJ, Windhager T, Holzer H, Leitner G, Muller W. FK 506 and successful pregnancy in a patient after renal transplantation. Transplant Proc. 1998;30(1):163. doi: 10.1016/s0041-1345(97)01220-7. [DOI] [PubMed] [Google Scholar]
- 78.French AE, Soldin SJ, Soldin OP, Koren G. Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother. 2003;37(6):815. doi: 10.1345/aph.1C312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardiner SJ, Begg EJ. Breastfeeding during tacrolimus therapy. Obstet Gynecol. 2006;107(2 Pt 2):453. doi: 10.1097/01.AOG.0000164052.66219.c7. [DOI] [PubMed] [Google Scholar]
- 80.Chusney GD, Bramham K, Nelson-Piercy C, et al. Tacrolimus monitoring during breastfeeding in neonates of transplant recipients. Ther Drug Monit. 2011;33(4):476. [Google Scholar]
- 81.Gouraud A, Bernard N, Millaret A, Bruel M, Paret N, Vial T. Serum level of tacrolimus in of breastfeed infant and long term follow-up. Fundam Clin Pharmacol. 2011;25:103. [Google Scholar]
- 82.Ward RM, Bates BA, Benitz WE, et al. The transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776. doi: 10.1542/peds.108.3.776. [DOI] [PubMed] [Google Scholar]