Table 1.
Cytotoxicity and antiviral activity of Subtilosin, Aciclovir (ACV) and Foscarnet (FOS) against HSV-1.
| Compound (MW) |
Structural Formula |
IUPAC name | Virus | CC50 (µg/ mL) |
EC50(µg/ mL) |
SI |
|---|---|---|---|---|---|---|
| Subtilosin * (3398.9) |
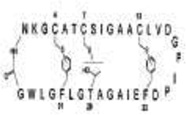 |
- | HSV-1 tk+ | 314 | 9.6 | 33 |
| HSV-1 tk deficient | 314 | 10 | 31 | |||
| FOS (300.04) |
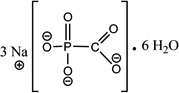 |
phosphonoformic acid trisodium salt hexahydrate |
HSV-1 tk+ | 210 | 15 | 14 |
| HSV-1 tk deficient | - | - | - | |||
| ACV (225.2) |
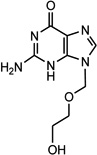 |
9-(2- hydroxyetoxymetyl) guanine |
HSV-1 tk+ | 67 | 0.07 | 957 |
| HSV-1 tk deficient | 67 | 6.4 | 10.4 | |||
CC50 compound concentration required to reduce cell viability by 50%, as determined by the MTT method.
EC50: compound concentration required to reduce virus yield by 50%.
SI (selectivity index): ratio CC50 / EC50.
Structural formula of subtilosin reproduced from Marx et al. [32].
