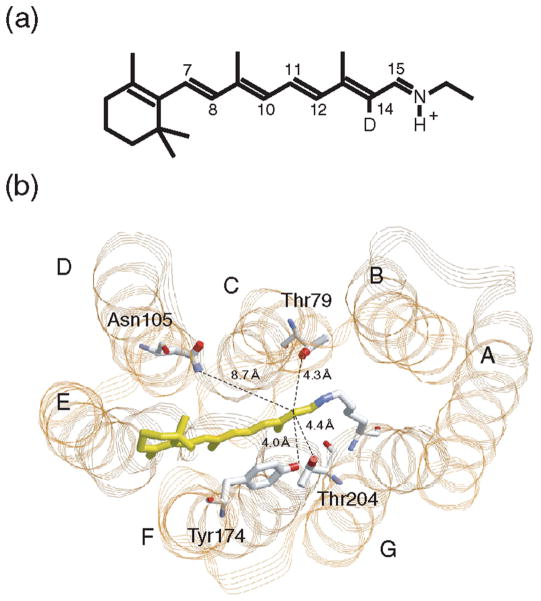
Figure 1.
(a) Chromophore molecule of SRII and BR. All-trans-retinal is bound to a lysine residue via a protonated Schiff base linkage. In this study, the C14 atom is deuterated. (b) X-ray crystallographic structure of SRII (PDB entry 1JGJ) in the extracellular-to-cytoplasmic view. Highlighted are the retinal chromophore (yellow) and residues that are mutated in this study. The distance from the C14 atom to the phenol oxygen of Tyr174 is 4.0 Å, to the hydroxyl oxygen of Thr204 is 4.4 Å, to the hydroxyl oxygen of Thr79 is 4.3 Å, and to the side chain nitrogen of Asn105 is 8.7 Å (7, 12). The OH group of Thr204 is within hydrogen bonding distance (3.2 Å) of the OH group of Tyr174.