Abstract
Oral fluid (OF) offers a non-invasive sample collection for drug testing. However, 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) in OF has not been adequately characterized in comparison to plasma. We administered oral low (1.0 mg/kg) and high (1.6 mg/kg) dose MDMA to 26 participants and collected simultaneous OF and plasma specimens for up to 143 h after dosing. We compared OF/plasma (OF/P) ratios, time of initial detection (tfirst), maximal concentrations (Cmax), time of peak concentrations (tmax), time of last detection (tlast), clearance, and 3,4-methylenedioxyamphetamine (MDA) to MDMA ratios over time.
For OF MDMA and MDA, Cmax was higher, tlast was later, and clearance was slower compared to plasma. For OF MDA only, tfirst was later compared to plasma. Median (range) OF/P ratios were 5.6 (0.1-52.3) for MDMA and 3.7 (0.7-24.3) for MDA. OF and plasma concentrations were weakly but significantly correlated (MDMA R2= 0.438, MDA R2= 0.197, p<0.0001). Median OF/P ratios were significantly higher following high dose: MDMA low 5.2 (0.1-40.4) and high 6.0 (0.4-52.3) (p<0.05); MDA low 3.3 (0.7-17.1) and high 4.1 (0.9-24.3) (p<0.001). There was large inter-subject variation in OF/P ratios. MDA/MDMA ratios in plasma were higher than those in OF (p<0.001), and MDA/MDMA ratios significantly increased over time in OF and plasma.
MDMA and MDA concentrations were higher in OF than in plasma. OF and plasma concentrations were correlated, but large inter-subject variability precludes estimation of plasma concentrations from OF.
Keywords: Toxicology; 3,4-methylenedioxymethamphetamine; MDMA; Oral fluid
INTRODUCTION
Sympathomimetic amine stimulants are among the most common illicit drugs in the world. Worldwide, an estimated 11-28 million people aged 15-64 consumed 3,4-methylenedioxymethamphetamine (MDMA) or its analogs in 2011 [1]. In 2011, 12% of 19- to 30-year-old Americans and 8% of 12th graders reported ever trying “ecstasy”, while past year use is 5% for 12th graders and 2% for 29- to 30-year olds. The 2007 National Roadside Survey of Alcohol and Drug Use by Drivers detected overall stimulants in 3.3% of nighttime drivers’ oral fluid (OF) or blood [2], second in prevalence to cannabis. In Victoria, Australia, MDMA was detected in 0.8% of injured drivers at a 20 μg/L cutoff in blood [3].
OF is gaining importance in drug testing, including in drug treatment, workplace, pain management, and driving under the influence of drugs (DUID) settings, because collection is noninvasive, easily observed, and has little potential for adulteration. Recently, we documented MDMA and metabolite pharmacokinetics in expectorated OF following controlled oral 1.0 and 1.6 mg/kg MDMA administration in 29 MDMA users [4]. Only MDMA and its metabolite 3,4-methylenedioxyamphetamine (MDA) were identified in OF; 4-hydroxy-3-methoxymethamphetamine (HMMA) and 4-hydroxy-3-methoxyamphetamine (HMA) metabolites were not detected. MDMA appeared to inhibit its own metabolism with much higher median Cmax for the high 1.6 mg/kg dose than the lower 1.0 mg/kg dose in proportion to the dose ratio. Median t1/2 was longer for the high dose (7.4 h) than the low (4.6 h) MDMA dose. OF MDMA and MDA concentrations should be greater than plasma concentrations due to the lower pH of OF compared to plasma, leading to ion trapping of the basic drug in OF.
We previously published MDMA plasma pharmacokinetics for 17 subjects [5]. Here we provide data for 26 participants, enabling additional testing of differences between low and high doses of MDMA, as well as the time course of OF/P ratios after controlled oral dosing.
There are few data on MDMA OF/P ratios; only two controlled administration studies and one self-administration study provide OF/P ratios and a comparison of OF and plasma pharmacokinetics [6-9]. However, these studies only documented OF/P ratios up to 24 h after dosing, when the average OF concentration was 126.2 μg/L, and time of last detection could not be compared between OF and plasma [9].
We evaluated OF and plasma MDMA and metabolites concentrations in 26 participants following controlled oral low (1.0 mg/kg) and high (1.6 mg/kg) dose MDMA administration for up to 143 h after dosing, to compare time of initial detection, peak concentrations, and duration of detection. These data will improve OF MDMA interpretation in clinical, drug treatment, law enforcement, and workplace drug testing programs.
MATERIALS AND METHODS
Reagents
Racemic mixtures of AMP-d0, MAMP-d0, MDMA-d0, MDA-d0, MDEA-d0, HMMA-d0 (plasma analysis), AMP-d11, MAMP-d14, MDMA-d5,MDA-d5, and MDEA-d6 were obtained from Cerilliant Corp. (Round Rock TX, USA). Racemic HMMA-d0 (OF analysis) and HMA-d0 was purchased from Lipomed, Inc. (Cambridge, MA, USA), p-hydroxymethamphetamine (pholedrine), ACS reagent grade Tris [hydroxymethyl] aminomethane base, Tris [hydroxymethyl] aminomethane hydrochloride, triethylamine (99.5% purity), and GC grade n-heptane from Sigma (St. Louis, MO, USA). ACS reagent-grade monobasic and dibasic potassium phosphate, hydrochloric acid (HCl), sodium hydroxide, acetic acid, isopropanol, ammonium hydroxide, and ethyl acetate (HPLC grade) were purchased from JT Baker (Phillipsburg, NJ, USA). HPLC-grade methanol was obtained from Fisher Scientific and heptafluorobutyric acid anhydride (HFAA) from Pierce Chemical Co. (Rockford, IL, USA). Styre Screen™ DBX solid-phase extraction (SPE) columns and fritted filters (10_m pore, 4mL reservoir volume) used in preparing oral fluid samples for solid phase extraction were purchased from United Chemical Technologies (Bristol, PA, USA). SPEC C18AR/MP1, 3mL reservoir/30 mg bed mass, mixed mode monolithic silica disc solid phase extraction columns were purchased from Varian Inc. (Lake Forest, CA, USA).
Human Participants
Healthy male and female participants provided written informed consent for this National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) Institutional Review Board-approved study. Participants were recruited by television, radio, and newspaper advertisements, flyers, and word of mouth; provided written informed consent; and were compensated for their time and inconvenience. Inclusion criteria were 18 to 40 years of age, lifetime consumption of at least five tablets of ecstasy, and at least one in the 90 days prior to screening. Female participants were required to utilize a reliable form of birth control or abstain from sexual intercourse throughout the study. A serum pregnancy test was administered at the screening visit and urine pregnancy tests were performed on the morning of each study session.
All participants received a comprehensive medical and psychological evaluation, including drug use history and physical examination, clinical laboratory tests, 12-lead electrocardiogram with 3-minute rhythm strip, Symptom Checklist-90-Revised (SCL-90R), and computer-administered screening version of the Structured Clinical Interview for the Diagnostic & Statistical Manual of Mental Disorders IV (DSM-IV). Exclusion criteria included: nursing and pregnant women; current medical condition or history of neurological illness, e.g., positive HIV serology test, positive Fluorescent Treponemal Antibody Absorption Test (FTA-ABS) confirmatory test for syphilis, head trauma with loss of consciousness for >3 min, stroke, central nervous system tumor, encephalitis or other CNS infection, multiple sclerosis or other demyelinating disease, epilepsy, movement disorder, or migraine headaches severe enough to require treatment; current axis I psychiatric diagnosis other than abuse or dependence on nicotine, cannabis or MDMA (including ethanol dependence); recent (within 30 days of MDMA administration) ingestion of a CYP2D6 or CYP3A4 inhibitor or CYP3A4 inducer; systolic blood pressure (BP) >135 mm Hg, diastolic BP >85 mm Hg, or heart rate >100 beats/min after 5 min rest; total cholesterol >250 mg/dL if older than 30 years; hemoglobin <12.5g/100 mL (male) or <12g/100 mL (female); clinically significant abnormal electrocardiogram; serum transaminase levels > three times normal.
Study Design
Participants had two options for study participation: one continuous 23-day residential stay with three dosing sessions, or three separate residential stays, each with a single dosing session, separated by at least one week and completed within a year. At each dosing session, participants ingested either placebo (0 mg/kg), low (1.0 mg/kg) or high (1.6 mg/kg) dose MDMA (Lipomed, Arlesheim, Switzerland) in a randomized, counterbalanced, double-blind design. Active drug was given as the hydrochloride salt, and placebo was given as lactose. Both study medications were packaged in size 0 Torpac® capsules (Fairfield, New Jersey, USA). A maximum 150 mg dose was given to the two participants (participants P and W) weighing more than 93.75 kg.
Participants entered the residential unit ≥12 h before dosing and remained for 1 to 7 days after dosing. Urine samples collected at entry were screened for benzodiazepines, cocaine, amphetamines, cannabis, opiates, phencyclidine (PCP), and barbiturates with Triage® 7 drugs of abuse panel (Biosite, Inc. San Diego, CA). Negative results were required for all drugs except amphetamines and cannabis. Participants completing the study with separate stays were re-evaluated before each stay to verify continued eligibility. The morning after admission, participants ate a light breakfast. Females with reproductive potential were given a urine pregnancy test. Baseline measures, biologic specimens, and a 12-lead electrocardiogram were collected. Participants then ingested study medication while seated. Dosing sessions were separated by at least 1 week. After dosing, participants remained seated and were monitored by medical staff for 3 h or until systolic BP, diastolic BP, and heart rate returned to within 20% of pre-dose levels (or heart rate to <95 beats/min), whichever occurred later. Plasma and expectorated OF were collected at −0.25, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, 4.5, 5.0, 7.0, 9.0, 11, 13, 15, 23, 29, 34, 39, 47, 71, 95, 119, and 143 h.
Specimen Collection
Whole blood was collected into sodium heparin (green-top) vacutainers and stored on ice up to 2 h. Samples were centrifuged at 4°C at 3000 RPM for 10 minutes for separation of plasma. Samples were stored frozen at −20°C until analysis. OF samples were collected by expectoration into polypropylene tubes.
Plasma Analysis
Plasma MDMA, MDA, HMMA, and HMA were quantified according to a previously published two-dimensional GCMS method with slight modifications [10]. Briefly, 1 mL plasma was diluted with 1 mL 0.5 mol/L HCl and incubated at 100°C for 40 min. After cooling, 1 mL 0.1 mol/L phosphate buffer (pH=6.0) and 50 μL 10 mol/L NaOH was added and samples centrifuged. Supernatant was decanted onto pre-conditioned polymeric Styre Screen DBX SPE columns and washed with deionized H20, 0.1 mol/L acetic acid and methanol. Dried columns were eluted with ethyl acetate:isopropanol:ammonium hydroxide (90/6/4, vol/vol/vol). Methanolic HCl (15 μL, 0.12 mol/L) was added to each extract prior to evaporation under nitrogen at 35 °C. Residues were reconstituted in 100 μL 0.5 mol/L triethylamine in heptane and derivatized with 10 μL HFAA at 60 °C for 20 min. After cooling, samples were back-extracted with 200 μL phosphate buffer (pH=7.4). The organic layer was injected onto an Agilent 6890 GC configured with a microfluidic Deans switch and flame ionization detector (FID) and interfaced to an Aglient 5973 mass selective detector (2D-GCMS). Recoveries were >85%. Linear ranges were 5-100 μg/L for MDA and HMA and 5-400 μg/L for MDMA and HMMA. R2 were >0.997, analytical bias was between 85.6-107.2% of target concentration, and coefficients of variation for interassay imprecision were ≤6.7% for all analytes (n=20 for each analyte).
Oral Fluid Analysis
Expectorated OF MDMA, MDA, HMMA, and HMA were quantified according to a previously published GCMS method [11], with a few modifications. Briefly, expectorated OF was centrifuged at 2000 × g for 6 min. A 400 μL aliquot was transferred to a 10 μm fritted filter reservoir inside a glass test tube. Samples were vortexed, diluted with 2 mL 0.1 M potassium phosphate buffer (pH=6.0), and centrifuged through filters at 1000 × g for 5 min. Filtrates were decanted onto preconditioned SPEC C18AR/MP1 columns and washed with acetic acid and methanol. Dried columns were eluted with ethyl acetate:methanol:ammonium hydroxide (78:20:2, v/v/v). Methanolic HCl (15 μL, 0.12 mol/L) was added to each extract prior to evaporation under nitrogen at 35 °C. Residues were reconstituted in 100 μL 0.05 mol/L triethylamine in heptane and derivatized with 10 μL HFAA at 60 °C for 20 min. After cooling, 200 μL 0.05 M Tris buffer (pH=7.4) was added and the organic layer injected onto the GCMS.
Injection volume was reduced to 1 μL to prevent saturation of the detector at elevated concentrations without sacrificing signal response at the limit of quantification (LOQ). Two calibration curves, utilizing a 1/x2 weighted least squares model, were established to encompass the wide range of drug concentrations. Low linear ranges were 5-500 μg/L for MDMA and MDA and 10-500 μg/L for HMA and HMMA; high linear ranges were 500-4000 μg/L for all analytes. R2 were >0.993 and recoveries were >85%. Analytical bias was 87.1-104.0% of target concentration, and coefficients of variation for interassay imprecision were ≤6.8% for all analytes (n=24 for each analyte).
Data Analysis
Statistical analyses were conducted with SPSS 13.0 for Windows. Visual inspection of data and evaluation by Kolmogorov-Smirnov tests indicated non-normal data distribution. Noncompartmental maximal concentration (Cmax), time to maximal concentration (tmax), half-life (t1/2), time of first detection (tfirst), and time of last detection (tlast), as well as MDA/MDMA ratios, were compared with Wilcoxon signed rank test. Cutoffs utilized for MDMA tlast included the analytical limit of quantification (5 μg/L), the Talloires cutoff (20 μg/L), the DRUID (Driving Under the Influence of Drugs, Alcohol and Medicines) cutoff (25 μg/L) and the proposed SAMHSA (Substance Abuse and Mental Health Services Administration) cutoff (50 μg/L) [12-14]. There are no defined cutoffs for MDA; therefore, evaluated cutoffs were 10 and 20 μg/L. Higher cutoffs of 25 and 50 μg/L were not evaluated for MDA because MDA plasma concentrations never exceeded these cutoffs. Least-squares regression analysis in Prism Version 5.02 (Graphpad Software Inc) was employed to evaluate concentrations and MDA/MDMA ratios. OF/P ratios for MDMA and metabolites were determined in simultaneously collected specimens. OF/P ratios were only calculated at time points when analytes were quantifiable in both matrices. Dose effects on OF/P were evaluated by Mann-Whitney tests. Significance was attributed at p<0.05 (two-tailed) for all statistical tests.
RESULTS
Human Participants
Twenty-six volunteers (16 males, 10 females) ages 18 to 35 years participated in the study (Table 1). Participants were 73% African-American, 23% Caucasian, and 4% unknown or mixed. Eight participants chose a continuous 23-day residential stay and provided all OF collections. Two participants completed only 2 of 3 dosing sessions (one as a continuous stay and one as two separate sessions). The other 16 participants completed all three sessions as separate stays and underwent fMRI scanning from 1.5 to 4 h after dosing to evaluate MDMA brain activity, precluding OF collection during this time and reducing available data for some pharmacokinetic analyses.
Table 1.
Demographics and self-reported 3,4-methylenedioxymethamphetamine (MDMA) use characteristics for 26 adult MDMA users.
| Participant | Race and ethnicity |
Gender | Age | BMI | MDMA use in last 14 days* |
Average MDMA tablets per month in the previous 3 months* |
|---|---|---|---|---|---|---|
| A | B | F | 22.2 | 28.2 | 10 | 16 |
| B | B | M | 23.9 | 29.5 | 1 | 28 |
| C | B | M | 24.7 | 23.7 | 1 | 3 |
| D | W | M | 35.0 | 24.4 | 2 | 1 |
| E | B | M | 19.6 | 26.5 | 2 | 12 |
| F | B | F | 20.1 | 24.1 | 1 | 4 |
| G | U & H | M | 19.0 | 22.4 | 1 | 2 |
| H | B | M | 22.4 | 21.9 | 3 | 1 |
| I | W | F | 19.5 | 18.6 | 2 | 36 |
| J | B | F | 26.1 | 34.2 | 5 | 4 |
| K | B | M | 18.4 | 24.4 | 1 | 2 |
| L | B | F | 27.6 | 23.3 | 6 | 8 |
| M | B | F | 23.3 | 19.1 | 0 | 32 |
| N | B | F | 22.0 | 18.0 | 1 | 8 |
| O | B | F | 20.1 | 26.4 | 2 | 2 |
| P | B | M | 20.1 | 27.3 | 8 | 12 |
| Q | W | M | 20.5 | 28.2 | 1 | 4 |
| R | W | M | 18.6 | 21.0 | 0 | 2 |
| S | B | M | 21.2 | 24.8 | 0 | 4 |
| T | B | F | 20.2 | 28.2 | 4 | 12 |
| U | B | M | 28.3 | 28.1 | 4 | 8 |
| V | B | M | 28.2 | 25.0 | 1 | 1 |
| W | B | F | 24.3 | 35.8 | 6 | 24 |
| X | W | M | 18.5 | 19.7 | 1 | 6 |
| Y | W | M | 21.9 | 24.4 | 1 | 2 |
| Z | B & H | M | 24.9 | 31.6 | 1 | 3 |
|
| ||||||
| Average | 22.7 | 25.3 | 2.6 | 8.8 | ||
| StdDev | 3.9 | 4.5 | 2.6 | 9.4 | ||
| Median | 22.0 | 24.6 | 1 | 4 | ||
| Min | 18 | 18 | 0 | 1 | ||
| Max | 35 | 36 | 10 | 36 | ||
B = Black or African American, W = White, U = Unknown, H = Hispanic or Latino
Data collected at screening
Comparison of OF and plasma MDMA and MDA pharmacokinetics
OF (N=1012) and plasma (N=1124) specimens were analyzed for MDMA, MDA, HMA, and HMMA. MDMA and MDA were quantified in 832 and 634 OF specimens and in 837 and 497 plasma specimens, respectively. Comprehensive OF (29 participants) and plasma pharmacokinetic analyses (17 participants) were presented previously [5,4]. Only 8 participants with a continuous 23-day stay are considered in this comparison. There were significant differences between plasma and OF for Cmax, tlast, and clearance for MDMA (Table 2) and for tfirst, Cmax, tlast, and clearance for MDA (Table 3).
Table 2.
Median (range) 3,4-methylenedioxymethamphetamine (MDMA) pharmacokinetic parameters for expectorated oral fluid and plasma after 1.0 and 1.6 mg/kg oral MDMA in 8 adult MDMA users.
| Pharmacokinetic Parameter | Plasma | Oral Fluid |
p value (between matrices) |
|---|---|---|---|
| Time of first detection (tfirst) | |||
| Low | 0.5 h (0.5-1.0) | 0.5 h (0.25-1.0) | 0.458 |
| High | 0.5 h (0.5-1.25) | 0.8 h (0.25-1.25) | 0.705 |
| Maximal Concentration (Cmax) | |||
| Low | 150 μg/L (132-218) | 1643 μg/L (1160-3382) | <0.05 |
| High | 291 μg/L (250-387)* | 4760 μg/L (2881-11,986)* |
<0.05 |
| Time of Cmax (tmax) | |||
| Low | 2.0 h (1.75-3.5) | 2.8 h (1.25-5.0) | 0.175 |
| High | 2.5 h (1.75-3.5) | 2.6 h (1.5-4.5) | 0.674 |
| Half-life (t1/2) | |||
| Low | 5.5 h (4.4-10.5) | 4.6 h (3.2-11.4) | 0.889 |
| High | 8.7 h (5.5-12.4)* | 7.4 h (6.0-13.4) | 0.575 |
| Time of last detection at 5 μg/L LOQ (tlast) |
|||
| Low | 29 h (23-39) | 36.5 h (29-47) | <0.05 |
| High | 43 h (34-47)* | 47 h (47-71)* | <0.05 |
| Time of last detection at 20 μg/L Talloires Cutoff (tlast) |
|||
| Low | 15 h (15-23) | 29 h (23-47) | <0.05 |
| High | 32 h (23-39)* | 43 h (29-47)* | <0.05 |
| Time of last detection at 25 μg/L DRUID Cutoff (tlast) |
|||
| Low | 15 h (13-23) | 23 h (23-34) | <0.05 |
| High | 32 h (23-34)* | 43 h (29-47)* | <0.05 |
| Time of last detection at 50 μg/L proposed SAMHSA Cutoff (tlast) |
|||
| Low | 12 h (9-15) | 23 h (13-29) | <0.05 |
| High | 19 h (15-23)* | 31 h (23-47)* | <0.05 |
| Clearance | |||
| Low | 620 mL/h/kg (470-884) |
112 mL/h/kg (35.9-170) |
<0.05 |
| High | 432 mL/h/kg (321-548)* |
57.7 mL/h/kg (20.3-92.6)* |
<0.05 |
Significant difference (p<0.05) between low and high doses for a given matrix
Table 3.
Median (range) 3,4-methylenedioxyamphetamine (MDA) pharmacokinetic parameters for expectorated oral fluid and plasma after 1.0 and 1.6 mg/kg oral 3,4-methylenedioxymethamphetamine (MDMA) in 8 adult MDMA users.
| Pharmacokinetic Parameter | Plasma | Oral Fluid |
p value (between matrices) |
|---|---|---|---|
| Time of first detection (tfirst) | |||
| Low | 0.8 h (0.5-1.2) | 1.1 h (0.8-1.8) | <0.05 |
| High | 0.8 h (0.5-1.5) | 1.0 h (0.5-1.5) | <0.05 |
| Maximal Concentration (Cmax) | |||
| Low | 7.2 μg/L (5.6-14.2) | 40.8 μg/L (23.1-151.3) | <0.05 |
| High | 13.5 μg/L (11.4- 23.3)* |
128.2 μg/L (50.5-403.2)* | <0.05 |
| Time of Cmax (tmax) | |||
| Low | 7 h (7.0-9.0) | 4.8 h (2.8-23) | 0.344 |
| High | 9 h (5.0-11.0) | 4.5 h (2.5-23) | 0.156 |
| Half-life (t1/2) | |||
| Low | 7.0 h (5.8-16.5) | 8.9 h (4.7-54.0) | 0.263 |
| High | 12.0 h (8.0-18.4)* | 8.1 h (6.9-22.8) | 0.208 |
| Time of last detection at 5 μg/L LOQ (tlast) |
|||
| Low | 15 h (9-15) | 26 h (23-47) | <0.05 |
| High | 26 h (23-39)* | 47 h (29-47)* | <0.05 |
| Time of last detection at 10 μg/L Cutoff (tlast) |
|||
| Low | 0 h (0-13) | 23 h (13-29) | <0.05 |
| High | 15 h (13-23)* | 39 h (23-47)* | <0.05 |
| Time of last detection at 20 μg/L Cutoff (tlast) |
|||
| Low | 0 h (0) | 12 h (3-29) | <0.05 |
| High | 0 h (0-13) | 23 h (23-29)* | <0.05 |
| Clearance | |||
| Low | 5656 mL/h/kg (4061-10,527) |
1904 mL/h/kg (530-2822) |
<0.05 |
| High | 4346 mL/h/kg (2468-5725)* |
1015 mL/h/kg (284-2359)* |
<0.05 |
Significant difference (p<0.05) between low and high doses for a given matrix
Comparison of OF and plasma MDMA and MDA concentrations
Of 992 paired OF-plasma specimens from 26 participants, 741 were positive for MDMA and 423 for MDA. OF concentrations were greater than those in simultaneously collected plasma specimens in 94.4% and 98.6% of samples for MDMA and MDA, respectively. Median (range) OF/P ratios were 5.6 (0.1-52.3) for MDMA and 3.7 (0.7-24.3) for MDA. The relationship between OF and plasma is illustrated in Figure 1. OF and plasma MDMA or MDA concentrations were significantly correlated, with R2= 0.438 and 0.197, respectively (all p<0.0001). Equations for the fitted lines were: y=11.1×-250 for MDMA and y=5.4×-5.9 for MDA. Median OF/P ratios were significantly higher following the high dose: MDMA low 5.2 (0.1-40.4) and high 6.0 (0.4-52.3) (p<0.05); MDA low 3.3 (0.7-17.1) and high 4.1 (0.9-24.3) (p<0.001).
Fig 1.
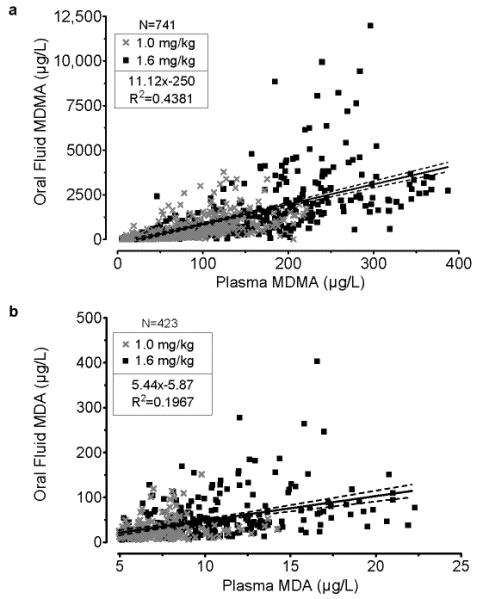
Oral fluid and plasma A) MDMA and B) MDA concentrations in all simultaneously collected paired-positive specimens collected −0.25 to 143 h after 1.0 and 1.6 mg/kg oral 3,4-methylenedioxymethamphetamine (MDMA) administration to 26 adult participants.
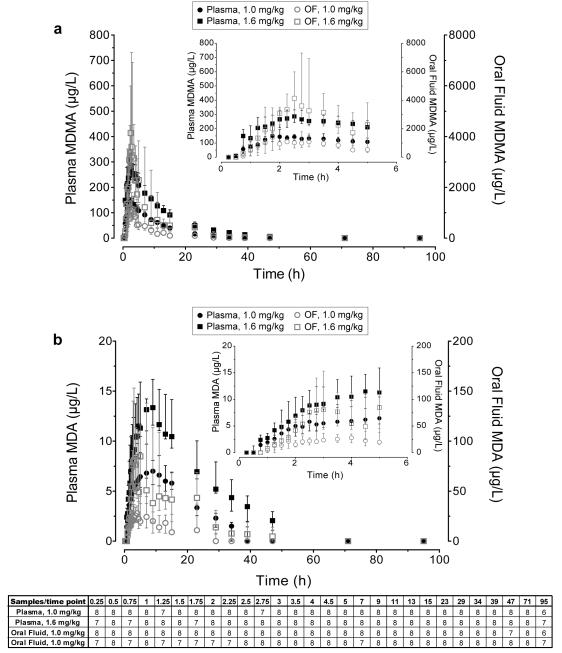
For the eight participants with a 23-day stay, OF MDMA and MDA concentrations were approximately 10-fold those of plasma and closely paralleled plasma concentrations over time (Figure 2). While there was large inter-subject variation, median OF/P ratios varied over time, peaking 1.3-2.5 h after oral MDMA administration. Fewer OF samples were collected immediately after MDMA dosing due to reduced salivary flow after psychostimulant administration.
Fig 2.
Median (interquartile range) A) MDMA and B) MDA oral fluid and plasma concentrations over time after 1.0 mg/kg and 1.6 mg/kg oral 3,4-methylenedioxymethamphetamine (MDMA) administration to 8 adult participants.
Buccal contamination appeared to occur in one participant’s samples after both low and high MDMA administrations. This individual was not one of the 23-day stay participants, thus, his data were not included in the tfirst determination. MDMA OF concentrations were 6507 and 7676 μg/L at 0.25 h after low and high doses, but dropped to 766 (low) and 407 μg/L (high) at 0.75 and 1.25 h, respectively, and increased thereafter. MDMA in this participant was not detected in plasma samples until 0.75 and 2 h after low and high doses. MDMA was not detected in baseline OF samples, and MDA was not detected in MDMA positive 0.25 h OF samples. It is possible that the participant cracked open the capsule before swallowing. Because MDMA was not detected in the first two corresponding plasma samples, these data were not included in OF/P ratio results.
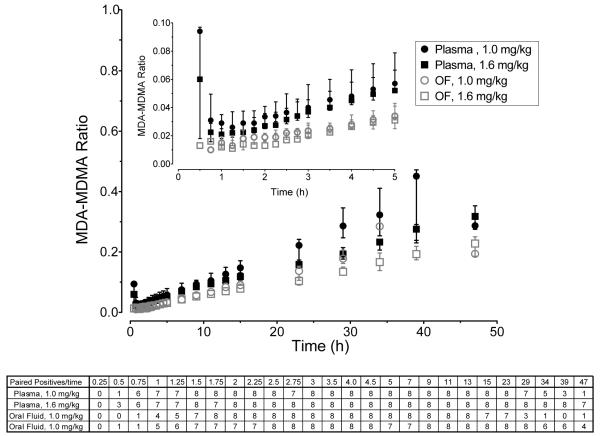
Metabolite-to-Parent Ratios
In the eight 23-day stay participants, MDA/MDMA ratios significantly increased over time in both matrices (Figure 3): R2 between MDA/MDMA ratios and time were 0.87, 0.92, 0.88, and 0.94 for plasma (low), plasma (high), OF (low), and OF (high), respectively (all p<0.0001). MDA/MDMA ratios in OF were correlated with those in plasma (R2=0.97, p<0.001). However, MDA/MDMA ratios in plasma were higher than those in OF (p<0.001) (Figure 3). The lower sample numbers at the start and end of the time course were due to reduced salivary flow following psychostimulant ingestion yielding low OF volume and lack of detection of concomitant MDMA and MDA at later time points, respectively.
Fig 3.
Median (interquartile range) oral fluid and plasma MDA-MDMA ratio in simultaneously collected paired-positive specimens collected 0.25 to 25 h after 1.0 and 1.6 mg/kg oral 3,4-methylenedioxymethamphetamine (MDMA) administration to 8 participants.
DISCUSSION
We present a thorough examination of the differences in disposition of MDMA and metabolites in human OF and plasma following controlled oral MDMA administration. This work is the first to document MDA OF/P ratios following MDMA administration. MDMA initially appeared in OF 0.25-1.0 h after dosing and peaked after 1.3-5 h, similar to plasma. However, MDA appeared slightly later in OF compared to plasma. For MDMA and MDA, OF Cmax was significantly greater than plasma Cmax. This is not surprising because both MDMA and MDA are basic drugs and are subject to ion trapping in OF due to its lower pH compared to blood. Furthermore, this is evidenced by the large 5.6 median OF/P ratio (0.1-52.3) for MDMA and 3.7 (0.7-24.3) for MDA (Figure 1), and the higher overall OF concentrations compared to plasma (Figure 2), indicating that these drugs partition into OF to a greater extent.
OF and plasma t1/2 were comparable for MDMA and MDA following low and high dose MDMA administration. This is inconsistent with a previous finding that MDMA t1/2 appeared shorter in OF [9]. However, in that study, no statistical analysis was performed to confirm the shorter t1/2 in OF. Time of last detection was significantly longer in OF for both MDMA and MDA. However, consideration of plasma HMMA (analytical LOQ 5 μg/L), which was not detected in OF, extended the detection window past that of MDMA in plasma or OF, to 71 h (71-95) and 95 h (71-119) following low and high doses, respectively. Clearance is significantly faster in plasma than OF for MDMA and MDA (Tables 2 and 3). This is most likely due to the ion trapping of the drug in the OF, leading to slow diffusion back into the plasma.
Median overall OF/P ratios were 5.6 (0.1-52.3) for MDMA and 3.7 (0.7-24.3) for MDA, with statistically significant correlations of OF and plasma concentrations. However, correlations were weak (R2=0.44 for MDMA and 0.20 for MDA), with large inter-subject variability, precluding direct prediction of plasma concentrations from OF levels. Samyn et al. reported OF/P ratios from 1-16.5 in 9 individuals who self-administered MDMA [6]. Others reported similar OF/P ratios of 0.8-22.4 following controlled administration of 75 mg MDMA in 25 mL orange syrup [7]. Mean ± SD maximal OF/P of 12±6 occurred at the first collection 1 h after dosing, and dropped to 4±3 by the last collection 4-5 h post-dose. Similar OF/P ratios were documented for the stereoisomers. Following controlled administration, R-(−)-MDMA OF/P ratios were 2.6-46.3, whereas S-(+)-MDMA ratios were 3.5-49.8 [8]. Navarro et al. also reported OF/P ratios of 1.2-32.2, with peak ratios at 1.5 h after a 100 mg MDMA dose (9). MDMA tmax were the same in OF and plasma. Half-life (t1/2) was longer in plasma (7.2 h) compared to OF (5.6 h). However, tfirst and tlast were not evaluated, as first collection was 1.5 h after dosing, and last collection was 24 h after dosing, when the average OF concentration was still 126.2 μg/L. Our 5.6 (0.1-52.3) MDMA OF/P ratio is consistent with previously reported ratios from other controlled drug administration studies and community cases [6-7,9,8]. We observed a large variation in OF/P ratio, indicating that plasma concentrations cannot be predicted from OF concentrations. Furthermore, OF/P ratio varied over time, peaking 1.3-2.5 h after MDMA administration. Similar peaks in OF/P ratios were reported elsewhere, occurring 1-1.5 h post-dose, although collection time points were less frequent than in our study [9,7].
OF/P ratios were significantly higher following the high (6.0, 0.4-52.3) compared to the low (5.2, 0.1-40.4) MDMA doses. We previously showed that MDMA inhibits its own metabolism [5]. We postulate that the non-linear pharmacokinetics creates a dynamic concentration gradient and that high MDMA plasma concentrations act to drive MDMA into the more acidic OF. Navarro et al. documented even more acidic OF following MDMA administration due to the lowered salivary flow, as well as a correlation between plasma concentration and OF/P ratio [9]. The higher plasma concentrations may prolong or exacerbate the lowered OF pH, leading to high drug accumulation in OF after the high dose.
In our study, one participant was suspected of having cracked the capsule prior to swallowing, leading to buccal contamination. Since MDMA is typically found in tablet form, we suspect that oral contamination may occur more frequently with self-administered drug. This may affect OF/P ratios for the first hour following drug ingestion.
MDA/MDMA ratios steadily increased over time in OF and plasma. MDA/MDMA in OF paralleled that in plasma, with a strong correlation, but ratios were significantly lower in OF. The delayed appearance of MDA in OF compared to plasma, lower OF/P ratios compared to MDMA, and the significantly lower MDA/MDMA ratio in OF compared to plasma all suggest that MDA does not enter the oral cavity as readily as MDMA. The only concomitantly measured MDMA and MDA pKa to our knowledge report conflicting pKa values [15]. MDMA pKa was estimated at 9.9 (derived from methamphetamine) [9] and MDA pKa is 9.67 [16]. Reported logP for MDMA and MDA are 1.68 and 1.38, respectively; the protein-bound fraction is 34-40% for both [15,17]. MDMA has a slightly higher pKa than MDA and thus, should be more ionized in plasma, slightly reducing its ability to cross into OF. On the other hand, MDMA is more lipophilic than MDA, suggesting that MDMA would distribute more rapidly into OF. Another factor that could explain the higher relative MDMA concentrations in OF is the 10 to 20-fold greater MDMA concentrations in plasma, yielding a greater concentration gradient and driving factor for MDMA to cross into OF. The MDA/MDMA ratio time course suggests that ratios might be useful for interpreting time of last use. However, the ratios presented here are based on a single MDMA administration and may not be representative of those after multiple doses. Furthermore, inter-subject variability precludes direct calculation of time of last use.
CONCLUSION
We document MDMA and MDA appearance in OF after controlled oral MDMA administration. MDMA and MDA concentrations were much higher in OF compared to plasma. OF and plasma concentrations were correlated, but large inter-subject variability precludes estimation of plasma concentrations from OF. Times of last detection were 24-48 h for MDMA and 12-47 h for MDA in OF. These results improve interpretation of OF tests in DUID cases and workplace and drug treatment programs
ACKNOWLEDGMENTS
We acknowledge the contributions of the clinical staffs of the National Institute on Drug Abuse, Intramural Research Program, and Behavioral Pharmacology Research Unit, Johns Hopkins Bayview Medical Center, as well as the Graduate Partnership Program, NIH and the Fondation Baxter et Alma Ricard. This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
References
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report 2011. Vienna: 2011. [Google Scholar]
- 2.Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, Brainard K, Moore C, Torres P, Berning A. 2007 National Roadside Survey of Alcohol and Drug Use by Drivers: Drug Results. National Highway Traffic Safety Administration Office of Behavioral Safety Research; 2009. [Google Scholar]
- 3.Drummer OH, Kourtis I, Beyer J, Tayler P, Boorman M, Gerostamoulos D. The prevalence of drugs in injured drivers. Forensic Science International. 2012;215(1-3):14–17. doi: 10.1016/j.forsciint.2011.01.040. doi:doi: 10.1016/j.forsciint.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Barnes AJ, Scheidweiler KB, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, Huestis MA. MDMA and Metabolite Disposition in Expectorated Oral Fluid After Controlled Oral MDMA Administration. Therapeutic Drug Monitoring. 2011;33(5):602–608. doi: 10.1097/FTD.0b013e3182281975. 610.1097/FTD.1090b1013e3182281975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma Pharmacokinetics of 3,4-Methylenedioxymethamphetamine After Controlled Oral Administration to Young Adults. Ther Drug Monit. 2008;30(3):320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samyn N, van Haeren C. On-site testing of saliva and sweat with Drugwipe and determination of concentrations of drugs of abuse in saliva, plasma and urine of suspected users. International Journal of Legal Medicine. 2000;113:150–154. doi: 10.1007/s004140050287. [DOI] [PubMed] [Google Scholar]
- 7.Samyn N, De Boeck G, Wood M, Lamers CTJ, De Waard D, Brookhuis KA, Verstraete AG, Riedel WJ. Plasma, oral fluid and sweat wipe ecstasy concentrations in controlled and real life conditions. Forensic Science International. 2002;128:90–97. doi: 10.1016/s0379-0738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 8.Peters FT, Samyn N, Kraemer T, Riedel WJ, Maurer HH. Negative-ion chemical ionization gas chromatography-mass spectrometry assay for enantioselective measurement of amphetamines in oral fluid: Application to a controlled study with MDMA and driving under the influence cases. Clinical Chemistry. 2007;53(4):702–710. doi: 10.1373/clinchem.2006.081547. [DOI] [PubMed] [Google Scholar]
- 9.Navarro M, Pichini S, Farre M, Ortuno J, Roset PN, Segura J, de La Torre R. Usefulness of saliva for measurement of 3,4-methylenedioxymehamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clinical Chemistry. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 10.Kolbrich EA, Lowe RH, Huestis MA. Two-dimensional gas chromatography/electron impact-mass spectrometry with cryofocusing for the sensitive, specific and simultaneous quantification of 3,4-methylenedioxymethamphetamine (MDMA, 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), and 3,4-methylenedioxyethylamphetamine (MDEA) in human plasma. Clinical Chemistry. 2008;54:379–387. doi: 10.1373/clinchem.2007.096800. [DOI] [PubMed] [Google Scholar]
- 11.Scheidweiler KB, Huestis MA. A validated gas chromatographic-electron impact ionization mass spectrometric method for methylenedioxymethamphetamine (MDMA), methamphetamine and metabolites in oral fluid. Journal of Chromatography B. 2006;835:90–99. doi: 10.1016/j.jchromb.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Walsh JM, Verstraete AG, Huestis MA, Morland J. Guidelines for research on drugged driving. Addiction. 2008;103:1258–1268. doi: 10.1111/j.1360-0443.2008.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pil K, Raes E, Neste TVd, Verstraete A. Toxicological analyses in the DRUID epidemiological studies: analytical methods, target analytes and analytical cut-offs. Paper presented at the The European Integrated Project DRUID; 2007.2007. [Google Scholar]
- 14.Bush DM. The U.S. Mandatory Guidelines for Federal Workplace Drug Testing Programs: current status and future considerations. Forensic Sci Int. 2008;174(2-3):111–119. doi: 10.1016/j.forsciint.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Garrett ER, Seyda K, Marroum P. High performance liquid chromatographic assays of the illicit designer drug “Ecstasy”, a modified amphetamine, with applications to stability, partitioning and plasma protein binding. Acta Pharm Nord. 1991;3(1):9–14. [PubMed] [Google Scholar]
- 16.Moffat AC, Osselton MD, Widdop B, Galichet LY. Clarke’s analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material. 3rd edn Pharmaceutical Press; London: 2004. [Google Scholar]
- 17.Barfknecht CF, Nichols DE. Correlation of psychotomimetic activity of phenethylamines and amphetamines with 1-octanol-water partition coefficients. J Med Chem. 1975;18(2):208–210. doi: 10.1021/jm00236a023. [DOI] [PubMed] [Google Scholar]