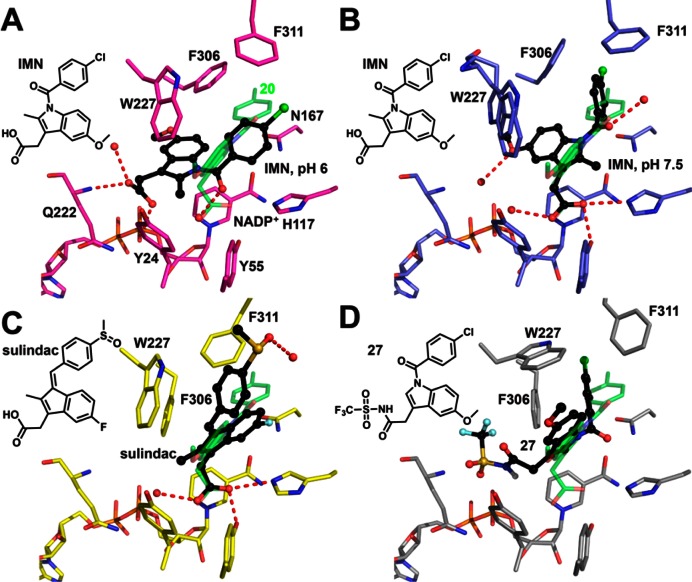
Figure 7.
Binding poses of indomethacin analogues in AKR1C3. (A) Indomethacin (IMN, black) at pH 6 (PDB ID: 1S2A), where the carboxylate group anchors the indole ring in the SP3 pocket by interacting with Q222 and a phosphate group of the pyrophosphate moiety of the cofactor. 2′-des-Methyl-indomethacin (20, green, PDB ID: 4DBW) is superimposed as a reference showing how the oxyanion site is occupied by the carboxylate group and the SP1 pocket is occupied by the p-chorobenzoyl ring. (B) Indomethacin (black) at pH 7.5 (PDB ID: 3UG8), where the carboxylate group is tethered to the oxyanion site and the p-chorobenzoyl ring penetrates the SP1 pocket. Two alternative conformations exist for Trp227, each with 50% occupancy. Note that the 5′-methoxy group on the indole ring points into SP3 so that the indole ring of indomethacin assumes a ∼120° angle with the indole ring of 2′-des-methyl-indomethacin. (C) Z-Sulindac (black, PDB ID: 3R7M), where the carboxylate group is anchored to the oxyanion site and the 4-methylsulfinyl group approaches the SP2 pocket. (D) The proposed fifth binding pose illustrated by compound 27 (black, generated by AutoDock Vina), where the N-(sulfonyl)acetamide group occupies the SP3 pocket, the p-chorobenzoyl ring occupies the SP1 pocket, and the indole ring almost overlaps with the indole ring of 2′-des-methyl-indomethacin. Noncarbon atoms, water, and hydrogen bonds are presented as described in Figure 6 where sulfur = yellow.