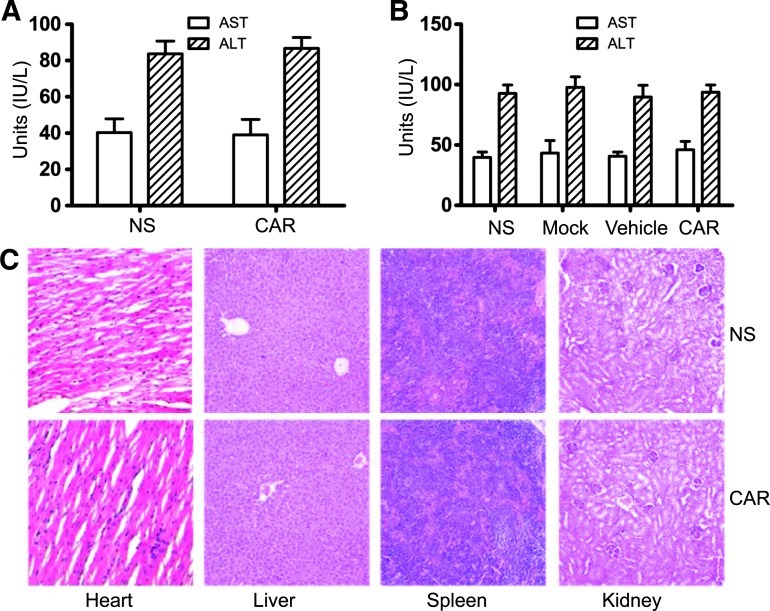
Figure 7.
Systemic toxicity evaluation of CAR-modified T cells. (A) Acute toxic effects induced by a single i.v. injection of CAR-modified T cells (2 x 107 cells per mouse). Forty-eight hours later, the mice were sacrificed and serum was separated. Levels of serum ALT and AST were measured. Bars, means ± SD. (B and C) Acute toxic effects induced by multiple i.v. injections of CAR-modified T cells. Some of the mice from the lung metastasis model were sacrificed 48 hours after the last cell administration, and several organs and serum were separated. (B) Levels of ALT and AST were measured using the same method as in A. Bars, means ± SD. (C) The heart, liver, spleen, and kidney tissues were used for morphologic analysis and H&E staining (original magnification, x100).