Abstract
Anthropogenic understorey fires affect large areas of tropical forest, yet their effects on woody plant regeneration post-fire remain poorly understood. We examined the effects of repeated experimental fires on woody stem (less than 1 cm at base) mortality, recruitment, species diversity, community similarity and regeneration mode (seed versus sprout) in Mato Grosso, Brazil. From 2004 to 2010, forest plots (50 ha) were burned twice (B2) or five times (B5), and compared with an unburned control (B0). Stem density recovered within a year after the first burn (initial density: 12.4–13.2 stems m−2), but after 6 years, increased mortality and decreased regeneration—primarily of seedlings—led to a 63 per cent and 85 per cent reduction in stem density in B2 and B5, respectively. Seedlings and sprouts across plots in 2010 displayed remarkable community similarity owing to shared abundant species. Although the dominant surviving species were similar across plots, a major increase in sprouting occurred—almost three- and fourfold greater in B2 and B5 than in B0. In B5, 29 species disappeared and were replaced by 11 new species often present along fragmented forest edges. By 2010, the annual burn regime created substantial divergence between the seedling community and the initial adult tree community (greater than or equal to 20 cm dbh). Increased droughts and continued anthropogenic ignitions associated with frontier land uses may promote high-frequency fire regimes that may substantially alter regeneration and therefore successional processes.
Keywords: disturbance, experimental burns, recruitment, savannah–forest boundary, succession, tropical forests
1. Introduction
The world's moist tropical forests are changing through the interactions of severe weather events, the rising concentration of atmospheric CO2, land use and fire, with important consequences for greenhouse gas emissions and biodiversity conservation [1–3]. These changes are predicted to intensify in the future as greenhouse gases accumulate further in the atmosphere, and as growing global demand for land-based commodities is met increasingly from tropical latitudes [4,5]. Fire is a particularly important factor in determining the magnitude of these changes in moist tropical forests because of the linkage it can create between land uses that provide abundant ignition sources and droughts that increase forest vulnerability to fire [6]. Large areas of moist tropical forests that have burned at intervals of several centuries [7,8] may be transformed to frequently burning scrub vegetation through positive feedbacks between land use and severe drought events [9]. Our understanding of current and future changes in tropical forests in response to the interacting influence of drought and land use is limited by the paucity of information on the effects of recurrent fires on forest regeneration.
Wildfires affect forest regeneration directly by killing stem tissues of seedlings and by heating the soil sufficiently to kill seeds and roots near the soil surface [10,11]. Fires in tropical forests also indirectly influence regeneration patterns by killing reproductive trees with thin bark [12–16], increasing resource availability near the ground (e.g. light, water and nutrients), and altering predation and herbivory patterns [17]. The fire frequency and intensity limits to woody plant regeneration are unknown for most tropical species. In addition, the timing of disturbance events relative to species-specific and community phenological patterns may be a critical factor in regeneration [18–21] as post-fire seed rain from surviving adults may find favourable conditions [22]. Also, fire-induced shifts to coppicing are a substantial [23], but generally an underappreciated pathway to forest recovery [24]. In tropical forests, where fire intervals have historically occurred on the order of centuries [7], intense or frequent fires may favour a distinctive suite of species from limited propagule sources and, in turn, alter forest dynamics and composition.
Studies of regeneration patterns after fire in closed-canopy tropical forests document an initial reduction in stem density and species diversity that is substantial and persistent after high-intensity burns, and rapid recovery after low-intensity fire events. In a moist aseasonal tropical forest of Indonesian Borneo, a sustained decline in seedling density and species diversity was observed 4 years after intense wildfires related to the 1997–1998 El Niño Southern Oscillation (ENSO) drought event [25]. However, after low-intensity burn treatments in a Bolivian closed-canopy dry forest, seedling density recovered after 18 months [11]. In a Venezuelan Amazon site, stem density recovered within 10 months and species richness within 22 months after simulated slash-and-burn events [10]. In all of these studies, fire increased stem mortality and therefore sprouting (i.e. coppicing) increased post-fire, but this capacity declined with more intense disturbances. The initial species establishing after fire in the Venezuelan Amazon were pioneers that survived in the seed bank, wind-dispersed invaders and sprouts—although combined cutting and burning killed sprouts [10]. In Bolivian forests, sprouting stems were less abundant than seedlings after experimental burns, yet attained greater height, had larger crown areas and experienced lower mortality than seedlings [11]. Fire in tropical forests may select for the subset of species that can sprout; for example, in the eastern Amazon, 41 per cent of surveyed tree species showed the capacity for vegetative sprouting after fire [26]. Overall, these studies lead us to expect fire frequency to be inversely related to stem density and species diversity, and positively related to the density of sprouting stems. There may also be a fire frequency threshold beyond which certain regeneration pathways, from seed or from sprouting, are diminished or effectively eliminated.
Although recent deforestation rates (2009–2012) in the southeastern Amazon have declined by up to 85 per cent of their historical rates from 1988 to 2008 [27], fire activity in the region has not diminished [28]. Less than a quarter of the transitional forests (original extent approx. 400 000 km2) between the cerrado (savannah) and high stature Amazon forest are expected to remain by 2050 [29]. Although other studies have assessed the effects of fire on regeneration dynamics, most of them lack pre-treatment data or fires that were large enough to represent the scale of actual escaped wildfires. In this study, we conducted a large-scale (150 ha), 6-year burn experiment designed to mimic the accidental understorey wildfires that escape into forest edges. We examined the effects of the burn frequency (annual, triennial) of low to moderate intensity fires (given natural inter-annual variability) on the mortality and recruitment of woody stems (less than 1 cm at base) through sprouting and seedling recruitment. We tested the hypotheses that increasing fire frequency would (i) increase mortality and decrease seedling and sprout regeneration, and thereby reduce overall stem density; (ii) reduce overall species diversity and alter community composition in two distinctive cohorts—initial colonizers and the stems that survived year to year; and (iii) promote sprouting over seedling regeneration.
2. Methods
(a). Site description and study design
Our focal site is located on Fazenda Tanguro, a privately owned agribusiness in Mato Grosso, Brazil, in the southern part of the Amazon basin (13°04’ 35’’ S, 52°23’ 08’’ W). Mean annual precipitation at the field site is 1739 mm, and dry season mean temperature is 25°C with 66 per cent relative humidity (average daily values). A severe dry season spans from May to September, when rainfall is less than 10 mm per month for three months followed by less than 50 mm per month for two months [30]. These experimental forests are found within the region's remaining transitional forest formation (above-ground biomass of trees and lianas, greater than or equal to 10 cm diameter at breast height (dbh), is 166±5 (s.e.) Mg ha−1 [14]), and exhibit relatively low plant species diversity (97 tree and liana species in stems greater than or equal to 10 cm dbh; the pre-treatment area sampled included 3.6, 16.5 and 150 ha for the 10–19.9, 20–39.9 and greater than or equal to 40 cm dbh size classes, respectively) with a high dominance of nine tree species (representing 50% of the importance value index [30]).
One 1.5 × 1.0 km (150 ha) experimental block with three treatment plots (0.5 × 1.0 km, 50 ha) was established within the property's legally protected forest reserves. Location was selected along a pasture edge within forest without known logging or recent fire, with less than 2 per cent slope, and containing at least 1000 m of forest extending around the experimental block. Three 50 ha treatment plots within the block include a control (B0), a plot burned twice (B2; burned in 2004 and 2007) and a plot burned five times (B5; burned in 2004, 2005, 2006, 2007 and 2009) over a total duration of 6 years (see [30] for additional study site description). The scale at which wildfires occur in the Amazon requires a large-scale ecosystem approach, which makes adequate experimental replication challenging [31]. A necessary limitation of this experiment is that we treat sampling within the 50 ha treatment plots as independent, which we acknowledge as a form of pseudoreplication that is often associated with experimental fires [32]. Key advantages of our planned burns, however, are that we were able to conduct intensive pre- and post-fire measures and compare these with an unburned control, following well-established procedures for large experimental manipulations without true replication [33].
(b). Regeneration monitoring
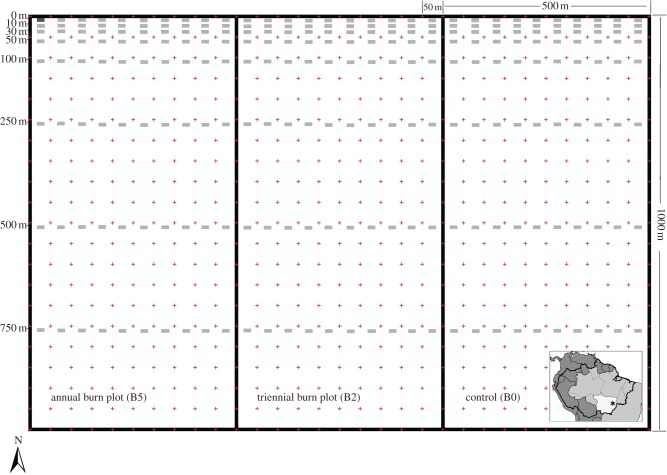
All woody plant stems less than 1 cm basal diameter (trees, lianas and shrubs) were tagged and identified to species within permanent 1 × 0.5 m subplots (0.5 m2) on eight transects varying in distance from the forest edge (0, 10, 30, 50, 100, 250, 500 and 750 m from the pasture-forest edge and parallel to the edge; figure 1). During the 6-year study period, 3908 stems were tagged and monitored. A stem was designated as dead if all above-ground plant tissue was dead (i.e. top-killed); owing to natural heterogeneity within the burned areas, not all stems were top-killed during each experimental fire. Along the eight transects, subplots were spaced 50 m apart. Before burn treatments, July 2004, two 0.5 m2 subplots were established at each transect per treatment plot (n = 16 per plot; total area sampled = 24 m2). In 2006, before the third burn, an additional eight subplots were established along five of the edge-parallel transects to increase sample size (increased to n = 56 per plot; total area sampled = 84.5 m2). In 2007, before the fourth burn, additionally eight subplots were established along the remaining three transects (n = 80 per plot; total area sampled = 120 m2). An initial and six annual censuses were conducted (2004–2010) within the month before that year's experimental fires.
Figure 1.
Experimental design and location of the 1 × 0.5 m subplots (grey rectangles) within each 50-ha treatment plot (crosses indicate junctions of trail network). (Online version in colour.)
New stems were tagged and classified as germinating from seed or sprouting at each census. To determine whether stems were from seed or sprout, nearby stems of the same species were carefully inspected (down to root structure) to determine the morphological habit of that species' seedlings and sprouts (e.g. root suckering was prevalent in Ocotea guianensis (Lauraceae)). Multiple sprouts (less than 1 cm at base) from an individual trunk base or rootstock were treated as separate stems. This study monitored stem mortality and regeneration, not individual mortality and regeneration, owing to the difficulty in non-destructively determining whether stems were originating from unique or shared root systems. Hereafter, the term ‘recruit’ refers to new individuals from seed, and ‘sprout’ to new stems from existing root structures. ‘Regeneration’ captures both sexual and vegetative modes of reproduction. Stems were identified to species using standardized nomenclature following the Missouri Botanical Garden TROPICOS database (www.tropicos.org). Stems that were unidentifiable to species or morpho-species were removed from the species-level analysis (individuals removed across all years from B0: n = 87; B2: n = 54; and B5: n = 59).
(c). Prescribed fires
Five annual experimental burns were conducted in August or in September (2004–2010), near the end of the dry season, when many escaped wildfires typically occur. Fires were set with kerosene drip torches; 10 km of fire lines per treatment plot were set between 09.00 and 16.00 during three to four consecutive days. Across all years, fires extinguished at night, and firelines were relit on subsequent days to complete the burn treatment. Because part of our trail network inhibited fire spread to the subplots, we applied firelines at the southern and eastern edge of the unburned subplots (on the same days as the plot-level experimental burns) to ensure that our treatment could at least reach the subplots; this focused ignition did not change the fuel load in the subplots themselves, and the climate conditions were similar to the overall plot treatment [30].
(d). Statistical analysis
(i). Annual mortality rates
Annual mortality rates were calculated based on the initial number of live stems, and the number of dead stems at the end of the measurement interval (1 year) and confidence limits (95% CI) were calculated based on the inverse F-distribution [34]. Hereafter, when we refer to all live stems, we are referring to surviving stems (less than 1 cm) from the prior years and any new stems that entered that year. The chi-squared test for two proportions was used to test whether the number of stems dying in each burned plot was significantly different from the control in a given year. The Kruskal–Wallis test was used to assess whether stem densities differed between the control and the burned plots in a given year.
(ii). Species richness in all regenerating stems by treatment
We compared species richness of total live stems (surviving and new seedlings and sprouts) in a given year by constructing stem-based rarefaction curves for each treatment plot and the control, based on Hurlbert's [35] formulation. These rarefaction curves plot the number of species sampled with the addition of each stem. A stem-based (rather than plot-based) analysis was used because it allows for different densities between treatments [36]. To construct these curves, we used the Vegan package [37] for R [38].
(iii). Yearly regeneration patterns of diversity and density in new stems
Relationships between plot treatment, the number of cumulative fires and distance from the forest edge to density and diversity of only new seedlings and sprouts entering each year were determined with structural equation models (SEMs), which allow for the determination of correlative and causal relationships [39]. Diversity of new stems was estimated by using the Shannon–Wiener index (H′) and Simpson's index (1−D). The Shannon–Wiener index is preferred for understanding the contribution of rare species to diversity, and Simpson's index describes species evenness [40]. The best-fit SEM, determined as maximum-likelihood goodness of fit, is presented (p > 0.05 indicates a good fit of the data). Significant parameter estimates have t-values less than 1.96, and non-significant relationships were maintained in the model if their inclusion led to an overall better fit of the model to the data. All response variables were log-transformed for normality, and analyses were completed in SAS software (SAS/STAT 9.1.3) with the Calis procedure using maximum-likelihood estimation (SAS Institute [41]).
Diversity (species richness, H′, and D) of current year regenerating stems was further analysed using split-plot repeated measures analyses of covariance (ANCOVA) with plot as the random effect (to deal with pseudoreplication within plots); all variables were log-transformed for normality, and predictor variables included the plot treatment, distance from forest edge and number of cumulative fires (the lowest Akaike information criterion (AIC) was used to select the best model). In these analyses, D is analysed without subtracting the value from unity; therefore greater values of D indicate less species evenness, and thus more dominants within subplots. Because the total sample area was small relative to each 50 ha treatment, the significance of the results was assessed by bootstrapping the effect of each predictor variable individually by resampling 10 000 times. These analyses included only new stems that entered during a given census year; stems surviving from one year to the next were omitted to compare current year regeneration between treatment levels. Analyses were performed with the mixed and Mult-test procedures [41]. Plot-level diversity (H′) in 2004 and 2010 (before the fire treatment began and at end of the experiment) was also bootstrapped 100 times without replacement in EstimateS [42]; these calculations were performed separately for seedlings and sprouts. Results shown are means for each level of the fire treatment with 95% CIs.
(iv). Community similarity across seedlings, sprouts and adults
To evaluate potential changes in community composition with repeated fires, the Morisita similarity index (MSI) was calculated for all live stems in 2004, before the fire treatment began, and in 2010, after five fires in the annually burned plot and two in the triennially burned plot. The index describes the similarity between multiple communities and is based on the Simpson concentration, so it is mainly sensitive to dominant species, but rare and undetected species are corrected for by bootstrapping 200 times. Pairwise comparisons between communities are also calculated with the MSI [43]. The analyses were performed with all identified stems using SPADE software [44]. For the 2010 analysis, 18, 12 and 13 unidentified stems were removed from the B0, B2 and B5 datasets, respectively. In addition, the MSI was calculated to compare the 2004 and 2010 community composition of seedlings, sprouts and living adults (greater than or equal to 20 cm dbh in six 20 × 500 m transects per level of the burn treatment; n ∼ 1000 individuals per treatment; shrubs were excluded; see [14,30] for sampling details).
3. Results
As described in the following sections, our results demonstrate that increasing fire frequency (i) increased mortality rates and reduced regeneration rates—more for seedlings than sprouts—leading to an overall decline in small (less than 1 cm) stem density; (ii) reduced species diversity with the loss of rare species, but maintained the most abundant species across plots by the end of the experiment; (iii) led to a divergence in the community composition of the seedling and adult communities; and (iv) shifted regeneration mode from seedling recruitment to increasing contributions from sprouting.
(a). Post-fire mortality and recruitment of seedlings and sprouts, and resulting live stem density
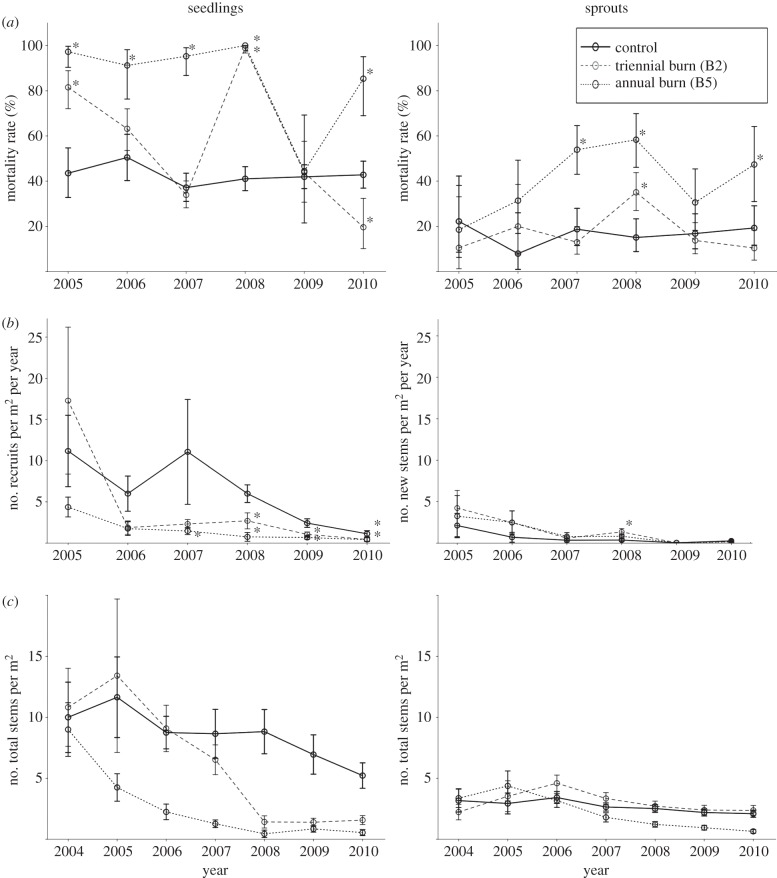
Annual mortality rates during the year directly following each experimental burn were 38–79% higher for seedlings (range: 82–100%) than for sprouts (range: 11–58%; figure 2a) in both annually and triennially burned plots. For seedlings in both burn treatment plots, mortality rates differed significantly from the control in the year following burns (chi-squared tests; p < 0.05). For sprouts, only after three annual burns and the second triennial burn were mortality rates significantly different from the control (chi-squared tests; p < 0.05). For both seedlings and sprouts, mortality rates were generally highest in the annually burned plot when compared with the triennially burned forest.
Figure 2.
For seedlings and sprouts: (a) annual mortality rate (% that died each year); (b) annual stem density (number of stems per m2 per year); and (c) stem density (number of live stems per m2, including surviving and new stems, at each annual census). Asterisks indicate whether the burned plot mortality (chi-squared test) and stem density (Kruskal–Wallis test) were significantly different from the control. Error bars are s.e.
More seedlings than sprouts regenerated annually in the unburned forest (figure 2b). Seedling recruitment significantly declined in the burned plots after three annual fires and two triennial fires, compared with the control (Kruskal–Wallis tests: p < 0.05). Sprout density post-fire did not differ from the control, except after four burns in 2008 when sprouting increased significantly (Kruskal–Wallis tests: p < 0.05). Sprouts represented an increased proportion of regeneration in the burned plots largely because of concomitant declines in seedling recruitment with additional experimental burns (figure 2b and electronic supplementary material, table S2). In B2 and B5, sprouts averaged 28 per cent and 37 per cent of new stems per year (2004–2010). By contrast, the control plot averaged 10 per cent regeneration by sprouting throughout the experiment, and new recruits germinating from seed comprised more than 80 per cent of new stems in all census years (see the electronic supplementary material, figure S1). Notably, a substantial decline in seedling and sprout regeneration occurred across all plots in 2009 and 2010. In 2010, only 56, 25 and 23 new stems were recorded in the control, B2 and B5, respectively (figure 2b).
Combined, these annual mortality and regeneration rates for seedlings and sprouts yielded total regeneration density for a given year (see the electronic supplementary material, figure S2). Total regeneration density before burning was comparable across plots, with an average of 13.2 (±3.1, s.e.), 13.1 (±3.3), 12.4 (±2.4) stems m−2 (less than 1 cm at base) initially inventoried in the control, B2 and B5 plots (see the electronic supplementary material, figure S2). In B2, recovery of regeneration density occurred within a year after the first burn and was sustained until the second triennial burn, then declined substantially. In B5, regeneration density decreased after each annual burn and exhibited the lowest regeneration density of all plots by 2010. In 2010, regeneration density was 7.3 (±1.1), 4.0 (±0.6) and 1.2 (± 0.3) in the control, B2 and B5 plots, respectively. Moreover, the burned plots had 54 per cent (B2) and 16 per cent (B5) fewer total stems (less than 1 cm) than the control plot (see the electronic supplementary material, figure S2).
(b). Species richness of all live stems post-fire
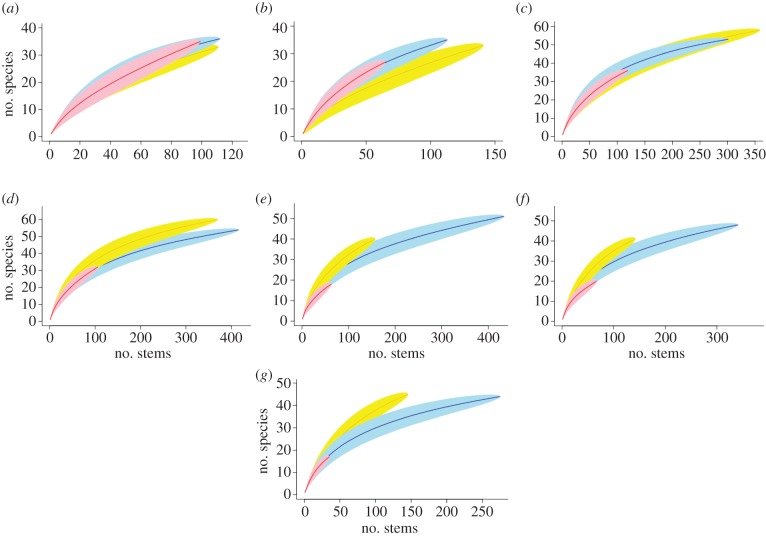
Similar stem-based rarefaction curves (±95% CI) of live stems for all plots at the onset of the experiment indicate that species richness was comparable across plots before the fire treatments. Total species richness was 36, 33 and 35 in the B0, B2 and B5 plots, respectively. Repeated burns reduced the total species number of all live stems, particularly in the annually burned plot (figure 3 and table 1). In B5, total species richness declined continually with each repeated annual burn, with notable species loss following the 2004 and 2007 burns (n = 27 and 18 species in B5 a year after those burns). By the 2010 census, species richness had not recovered in B5, but it did recover in B2. However, because rarefaction curves tend to converge at low abundances [45], these declines in species richness are not statistically distinguishable based on the stem-based rarefactions curves (figure 3; but see ANCOVA results for comparisons of plot rather than stem-level richness and diversity indices; tables 1–3).
Figure 3.
Stem-based rarefaction curves of all censused live stems in each year. Blue, control; yellow, triennial burn (B2); red, annual burn (B5). (a) 2004, (b) 2005, (c) 2006, (d) 2007, (e) 2008, (f) 2009 and (g) 2010.
Table 1.
Effects of fire, number of repeated fires and distance from the forest edge on current year species richness for all regenerating stems (seedlings and sprouts combined). The best-fit model is presented.
| AIC =−7288.2 | F | d.f. | p |
|---|---|---|---|
| fire treatment | 2.4 | 2/400 | 0.1 |
| number of years of repeated fires | 36.5 | 6/850 | <0.0001 |
| distance from edge | 40.0 | 1/266 | <0.0001 |
| treatment × years of repeated fire | 1.1 | 12/850 | 0.4 |
| treatment × distance from edge | 8.0 | 2/266 | 0.0004 |
| years of repeated fire × distance | 19.0 | 6/837 | <0.0001 |
| treatment × years of repeated fire × distance | 3.6 | 12/838 | <0.0001 |
Table 2.
Effects of fire, number of repeated fires, and distance from the forest edge on the Shannon–Wiener index for current year regenerating stems (seedlings and sprouts combined). The best-fit model is presented.
| AIC = 2818.0 | F | d.f. | p |
|---|---|---|---|
| fire treatment | 1.1 | 2/381 | 0.3 |
| number of years of repeated fires | 8.9 | 6/533 | <0.0001 |
| distance from edge | 30.0 | 1/286 | <0.0001 |
| treatment × years of repeated fire | 2.0 | 12/529 | 0.03 |
| treatment × distance from edge | 1.0 | 2/287 | 0.4 |
| years of repeated fire × distance | 1.2 | 6/492 | 0.3 |
| treatment × years of repeated fire × distance | 1.8 | 12/500 | 0.04 |
Table 3.
Effects of fire, number of repeated fires, and distance from the forest edge on the Simpson's index for current year regenerating stems (seedlings and sprouts combined). The best-fit model is presented.
| AIC = 1595.9 | F | d.f. | p |
|---|---|---|---|
| fire treatment | 1.3 | 2/182 | 0.3 |
| number of years of repeated fires | 0.7 | 6/250 | 0.6 |
| distance from edge | 6.4 | 1/119 | 0.01 |
| treatment × distance from edge | 2.9 | 2/120 | 0.06 |
(c). Effects of burn treatment, increasing fires and edge distance on subplot-level diversity and density of new stems
The subplot-level analyses (ANCOVA) of yearly regeneration diversity demonstrated that the number of cumulative fires and distance from the forest edge were important in determining both species richness and the Shannon–Weiner index (H′), which best captures rare species (tables 1 and 2). Richness and H′ declined with an increasing number of burns and increased with greater distance from the forest edge in all plots. Simpson's index (D), which best describes species evenness, also increased with distance from the forest edge (demonstrating a loss of evenness; table 3). Overall, the number of cumulative fires had strong effects on regeneration diversity and richness immediately following a burn. The bootstrapped analyses confirm these results, as the significance of the effects was not altered via resampling (see the electronic supplementary material, table S1).
Similarly, the SEM shows the burn treatment reduced both the diversity and density of regenerating stems each year, and increasing cumulative fires diminished diversity even more strongly (see the electronic supplementary material, figure S3). Regeneration density increased with distance from the forest edge, although distance did not affect the diversity indices (χ2 = 0.0005, d.f. = 1, p = 0.98; electronic supplementary material, figures S3 and S5). At the onset of the experiment, the density of all live stems increased with increasing distance from the forest–pasture edge (0–500 m) and was maintained in the control plots throughout the experiment. However, this effect diminished substantially with increasing fire frequency in both burned plots; by 2010, the interior forest subplots (250–750 m) had similar live stem densities compared with the edge subplots (0–100 m; electronic supplementary material, figure S5). At 750 m into the forest, stem density dropped almost fourfold and sixfold in B2 and B5, respectively, between 2004 and 2010.
When seedlings and sprouts are combined, the diversity (H′) of new stems entering each year is lower than the initial inventoried stems and is quite variable from year to year (figure 4). However, there is an overall decline in diversity (H′) with increased burning by the end of the experiment (figure 4). We also bootstrapped subplot-level H′ with separated seedlings and sprouts for current year stems. Pre-treatment seedling diversity was higher than sprout diversity across the forest, and, similarly to the stem-based rarefaction results, there were no detectable differences in diversity among plots (see the electronic supplementary material, figure S4). By the end of the experiment, diversity did not differ between levels of the fire treatment based on 95% CIs, although seedling and sprout diversity appear lowest in the annually burned plot (see the electronic supplementary material, figure S4).
Figure 4.
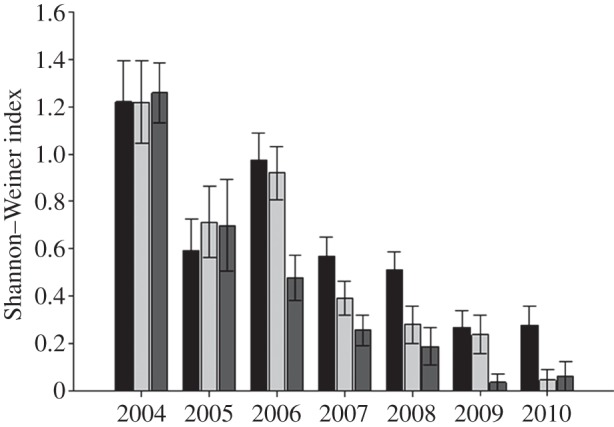
Shannon–Weiner index for all new entering stems (seedlings and sprouts combined) in each year. Note that 2004 represents the initial inventory of all existing stems. Black bars, control; light-grey bars, triennial burn; dark-grey bars, annual burn.
(d). Community similarity and rank abundance of seedlings and sprouts post-fire
In 2004, before the burning treatment was initiated, species composition of all inventoried stems in the three forest plots was relatively similar, based on the MSI, a multiple community similarity measure (three-way comparison, MSI = 0.76 ± 0.11 s.e.). In 2010, after two triennial and five annual burns, we observed remarkable similarity in seedling and sprout communities across the control and burned plots, despite substantial differences in stem density (seedlings, MSI = 0.95 ± 0.22; sprouts, MSI = 0.93 ± 0.17; figure 2c). In 2010, in the control plots, 195 seedlings were identified from 38 different species. In B2, there were 58 individuals from 22 species, whereas there were only 11 individuals from seven species in B5. Five of the seven species in the annually burned plots were shared with both the control and the triennially burned plots, and 14 species were common to the triennially burned and control plots. Only four species were found in all three treatment plots. Sprouts were much more common than seedlings in the burned plots. In 2010, 87 sprouts from 33 species were identified in B2, and 24 sprouts were identified from 11 species in B5. By contrast, the unburned plot had fewer sprouts than seedlings; there were 79 sprouts identified from 23 species. Within levels of the burn treatment, the only notable difference between seedling and sprout communities was in the annually burned plot, where the species composition of the two regeneration modes diverged (see the electronic supplementary material, table S2).
When community composition was analysed with all surviving stems (combining seedlings and sprouts) across levels of the burn treatment, the lower similarity index value reveals that many species have distinct modes of reproduction (MSI = 0.60 ± 0.07 s.e.). Even within the unburned forest, distinctive patterns emerged between suites of species that relied on seed for regeneration and those species that vegetatively reproduced from sprouts (see the electronic supplementary material, table S2).
The striking community similarity across plots reflects, in part, that the most abundant species are shared across plots (see the electronic supplementary material, table S3). The ranked relative abundance of the five most abundant species (combined seedlings and sprouts) in 2004, 2007 and 2010 demonstrates how persistent these species are, despite frequent burning. In 2010, Protium guianense (Burseraceae), Elachyptera floribunda (Celastraceae) and Myrcia multiflora (Myrtaceae) were among the five most abundant species (based on live stems) across all plots. The latter two species demonstrated increased sprouting with increased fire frequency; E. floribunda is a liana, whereas M. multiflora is found in the cerrado [46]. Over the duration of the experiment, of all E. floribunda stems that entered the annual censuses, 26 per cent, 33 per cent and 40 per cent were sprouts in the control, B2 and B5 plots, respectively. Of all M. multiflora stems that entered, 33 per cent, 62 per cent and 60 per cent were sprouts in the control, B2 and B5 plots. However, P. guianense reproduced predominantly via seed, with only 10 per cent, 10 per cent and 0 per cent of stems coming from sprouts in the control, B2 and B5 plots (see the electronic supplementary material, table S4). Across the experiment, 83 per cent of all observed species in the regeneration community demonstrated sprouting capacity (of the 71 species that had more than five inventoried stems); 12 species only reproduced by seed, whereas five only persisted by sprouting (see the electronic supplementary material, table S4).
High-fire frequency shifted the seedling and sprout communities away from the adult community composition, as seen when all live stems in 2010 (surviving and new) were compared with the adult communities of 2004 and 2010 by burn treatment (MSI = 0.47 ± 0.03; electronic supplementary material, table S5). By the end of the experiment, the B2 seedling community was more like the initial 2004 adult community than was the B5 seedling community. In addition, the seedling community in the unburned forest was equally similar to the adult communities in both 2004 and 2010. The identities of seedlings and sprouts differed more in the annually burned area than in the other two plots, indicating that a divergence in regeneration modes may contribute to a divergence in community composition (see the electronic supplementary material, table S5). Before any treatment, adults were more similar in composition than regenerating communities across plots (see the electronic supplementary material, table S5).
4. Discussion
Our results corroborate the overall hypothesis that understorey fires can alter early regeneration patterns of southeast Amazonian forests. Repeated fires not only substantially reduced stem density, but also shifted the predominant regeneration mode from seedling recruitment to relatively higher contributions from sprouting. After two and five fires within 6 years, the community similarity within the regenerating communities was comparable among treatments, but with increasing fire frequency there was a considerable decline in species richness, and loss of rare species. Moreover, the annual burn regime shifted the regenerating community composition away from the initial adult tree community composition that existed before any burn treatment.
Mortality rates were significantly higher in the re-census year immediately following experimental fires than in the control. Moreover, mortality rates were 38–79% greater for seedlings than for sprouts in both annually and triennially burned plots. Seedling recruitment declined significantly after three annual and two triennial burns, whereas sprout regeneration did not differ significantly from the control, except for increases detected only in 2006. Although stem density recovered after the first burn, combined higher mortality and lower seedling recruitment led to a significant reduction in stem density after repeated burns in B2 and B5. By 2010, burned plots had only 54 per cent (B2) and 16 per cent (B5) of the stems observed in the control. Moreover, stem density initially declined with proximity to edge, but this effect changed in the burned plots as burning reduced stem densities four- and sixfold even at the forest interior (750 m). The fire-induced canopy openness [30] created more edge-like conditions in the forest interior that also led to drier conditions, higher fire intensities and greater competition with invasive grasses [47]. Similar patterns of stem density recovery were observed within 18 months after a single fire event in Bolivian seasonally dry forests [11] and within 10 months following a slash-and-burn simulation in Venezuelan humid tropical forests [10]. Also similar to our findings, high-intensity or repeated fires substantially hindered successful tropical forest regeneration. Four years after an intense ENSO-related wildfire in Borneo, seedling and sapling density (stems less than 1.5 m in height) declined 75 per cent when compared with nearby unburned forests [25].
After two triennial and five annual burns, remarkable similarity in seedling (MSI = 0.95±0.22 s.e.) and sprout communities (MSI = 0.93±0.17) were observed across the control and burned plots. Given the MSI emphasizes dominant species this high similarity may simply reflect abundant species shared across plots. Notably, the annual burn did create a divergence between the seedling and adult communities (pairwise comparison = 0.18). The contrast between the regenerating and adult communities may reflect that overall mortality of stems (greater than or equal to 1 cm dbh) was 1.8 and 2.2 greater in B2 (5.8% yr−1) and B5 (7.0% yr−1) than in the control (3.2% yr−1) by 3 years after the initial treatment, but large, reproductive trees had a higher probability of survival post-fire [14]. Moreover, fire-induced mortality varied substantially by species [14,15]. These combined factors probably influenced seed production and sprouting behaviour across the burned plots.
Overall species richness declined in the stems that survived post-fire. B2 plot lost 12 species, whereas B5 plot lost 29 species that were present before the burn treatment, but not recorded in 2010. In seasonally dry forests of India, Saha & Howe [48] found a 30 per cent decrease in seedling species diversity with repeated annual burns. Moreover, B5 had 11 new species at low densities (less than four individuals) that were not recorded in 2004, but are often found in disturbed areas, e.g. Mabea fistulifera (Euphobiaceae), Tachigali vulgaris (Fabaceae, formerly Sclerolobium paniculatum) and Pyrostegia dichotoma (Bignoneaceae) [49,50]. By 2010, the most dominant species were P. guianense, E. floribunda and M. multiflora—a seed recruiter, sprouting liana and sprouting cerrado species—suggesting that some species are able to persist post-fire via seed production and others via vegetative reproduction.
Although the same dominant regenerating species are present across plots by 2010, regeneration by sprouting had increased considerably in the burned sites. This increase in sprouting is due to the fire-induced increase in top-kill. Across all years, sprouts averaged 10 per cent of the stems in the control, compared with 28 per cent in B2 and 37 per cent in B5. Increased sprouting post-fire has been observed in other tropical forests, but declines with increasing disturbance [10,11,16]. Repeatedly burned sprouts may not escape the ‘fire trap’ to move into less vulnerable size classes [51]. We documented that 83 per cent of the species in this study demonstrated sprouting capacity (see the electronic supplementary material, table S3). These sprouts could play an increasing role in tropical forest dynamics [52] if they are able to outcompete other forest species and invasive grasses by occupying the ‘persistence niche’ [53]. Moreover, our results demonstrate that high-fire frequency shifted the seedling communities away from the initial adult community composition (MSI = 0.47±0.03), suggesting that fire has altered the early seedling regeneration pathway.
5. Conclusion
Repeated burns resulted in significant increases in mortality and declines in regeneration, species richness and diversity for small stems (less than 1 cm). A key result documented here is that although resilience in regeneration capacity is evident after a single fire event, repeated fires substantially inhibit early regeneration of forest species, especially seedling recruitment. Our results imply that a period of frequent burns would eliminate regeneration of several species. And although the same species were dominant across levels of the fire treatment, sprouting increased in relative importance over seedling reproduction. This sprout-dominated regeneration may facilitate rapid recovery of tree cover in degraded forests, and thus should be considered in models developed to project future vegetation scenarios [24]. Alternatively, if sprouting does not result in eventual seed production, this altered regeneration pathway could yield a degraded forest. Moreover, if grasses from adjacent land uses outcompete sprouts, a fire-maintained alternative stable state dominated by pyrophilic grasses, herbs and shrubs may persist [54]. After 3 years of repeated fire, invasive grasses associated with the pasture edge were observed more than 25 m into the forest [55], and they penetrated further by 2010 [47]. Our experiment is simulating an extreme fire frequency (five burns in 6 years), but this scenario could be more representative if pyrophilic vegetation establishes concurrent with high land use ignitions. Continued empirical studies of post-fire forest regeneration dynamics across the seasonality gradient are critical to assessing the potential for Amazon forest dieback and replacement by an alternative vegetation state.
Acknowledgements
We are grateful to the Woods Hole Research Center and the Instituto de Pesquisa Ambiental da Amazônia for institutional support, in particular, to the field crew at the study site; the many visiting researchers and students for assistance in carrying out the experimental fires; N. Rosa for plant identification; R. Cury for species’ life-history information; and O. Carvalho for team management and acquiring permits. Special thanks to Grupo Amaggi, who invited this research to be conducted on their farm and provided infrastructure support. The comments of R. Chazdon and four anonymous reviewers helped improve an earlier version of this manuscript. Funding for this research was provided by the David and Lucile Packard Foundation, the Blue Moon Fund, the NSF Biocomplexity in the Environment (no. 0410315) and Ecosystems programmes (no. 0743703), and the NASA Earth Systems Land Use and Land Cover Change Program (no. NNX11AF08G and no. NNX07AK37H).
References
- 1.Malhi Y, Aragao L, Galbraith D, Huntingford C, Fisher R, Zelazowski P, Sitch S, McSweeney C, Meir P. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20 610–20 615 10.1073/pnas.0804619106 (doi:10.1073/pnas.0804619106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nepstad DC, Stickler CM, Soares B, Merry F. 2008. Interactions among Amazon land use, forests and climate: prospects for a near-term forest tipping point. Phil. Trans. R. Soc. B 363, 1737–1746 10.1098/rstb.2007.0036 (doi:10.1098/rstb.2007.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson EA, et al. 2012. The Amazon basin in transition. Nature 481, 321–328 10.1038/nature10717 (doi:10.1038/nature10717) [DOI] [PubMed] [Google Scholar]
- 4.Bowman MS, Soares-Filho BS, Merry FD, Nepstad DC, Rodrigues H, Almeida OT. 2012. Persistence of cattle ranching in the Brazilian Amazon: a spatial analysis of the rationale for beef production. Land Use Policy 29, 558–568 10.1016/j.landusepol.2011.09.009 (doi:10.1016/j.landusepol.2011.09.009) [DOI] [Google Scholar]
- 5.Macedo MN, DeFries RS, Morton DC, Stickler CM, Galford GL, Shimabukuro YE. 2012. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proc. Natl Acad. Sci. USA 109, 1341–1346 10.1073/pnas.1111374109 (doi:10.1073/pnas.1111374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nepstad D, et al. 2001. Road paving, fire regime feedbacks, and the future of Amazon forests. Forest Ecol. Manag. 154, 395–407 10.1016/S0378-1127(01)00511-4 (doi:10.1016/S0378-1127(01)00511-4) [DOI] [Google Scholar]
- 7.Bush MB, Silman MR, McMichael C, Saatchi S. 2008. Fire, climate change and biodiversity in Amazonia: a Late Holocene perspective. Phil. Trans. R. Soc. B 363, 1795–1802 10.1098/rstb.2007.0014 (doi:10.1098/rstb.2007.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanford RL, Saldarriaga J, Clark KE, Uhl C, Herrera R. 1985. Amazon rain-forest fires. Science 227, 53–55 10.1126/science.227.4682.53 (doi:10.1126/science.227.4682.53) [DOI] [PubMed] [Google Scholar]
- 9.Veldman JW, Putz FE. 2011. Grass-dominated vegetation, not species-diverse natural savanna, replaces degraded tropical forests on the southern edge of the Amazon Basin. Biol. Conserv. 144, 1419–1429 10.1016/j.biocon.2011.01.011 (doi:10.1016/j.biocon.2011.01.011) [DOI] [Google Scholar]
- 10.Uhl C, Clark K, Clark H, Murphy P. 1981. Early plant succession after cutting and burning in the upper Rio Negro region of the Amazon basin. J. Ecol. 69, 631–649 10.2307/2259689 (doi:10.2307/2259689) [DOI] [Google Scholar]
- 11.Kennard DK, Gould K, Putz FE, Fredericksen TS, Morales F. 2002. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. Forest Ecol. Manag. 162, 197–208 10.1016/S0378-1127(01)00506-0 (doi:10.1016/S0378-1127(01)00506-0) [DOI] [Google Scholar]
- 12.van Nieuwstadt MGL, Sheil D. 2005. Drought, fire and tree survival in a Bornean rain forest, East Kalimantan, Indonesia. J. Ecol. 93, 191–201 10.1111/j.1365-2745.2004.00954.x (doi:10.1111/j.1365-2745.2004.00954.x) [DOI] [Google Scholar]
- 13.Barlow J, Lagan BO, Peres CA. 2003. Morphological correlates of fire-induced tree mortality in a central Amazonian forest. J. Trop. Ecol. 19, 291–299 10.1017/S0266467403003328 (doi:10.1017/S0266467403003328) [DOI] [Google Scholar]
- 14.Balch JK, Nepstad DC, Curran LM, Brando PM, Portela O, Guilherme P, Reuning-Scherer JD, de Carvalho O. 2011. Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. Forest Ecol. Manag. 261, 68–77 10.1016/j.foreco.2010.09.029 (doi:10.1016/j.foreco.2010.09.029) [DOI] [Google Scholar]
- 15.Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE. 2012. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob. Change Biol. 18, 630–641 10.1111/j.1365-2486.2011.02533.x (doi:10.1111/j.1365-2486.2011.02533.x) [DOI] [Google Scholar]
- 16.Pinard MA, Putz FE, Licona JC. 1999. Tree mortality and vine proliferation following a wildfire in a subhumid tropical forest in eastern Bolivia. Forest Ecol. Manag. 116, 247–252 10.1016/S0378-1127(98)00447-2 (doi:10.1016/S0378-1127(98)00447-2) [DOI] [Google Scholar]
- 17.Massad TJ. In press Interactions between repeated fire, nutrients, and insect herbivores affect the recovery of diversity in the southern Amazon. Oecologia. 10.1007/s00442-012-2482-x (doi:10.1007/s00442-012-2482-x) [DOI] [PubMed] [Google Scholar]
- 18.Barlow J, Peres CA. 2005. Effects of single and recurrent wildfires on fruit production and large vertebrate abundance in a central Amazonian forest. Biodivers. Conserv. 15, 985–1012 10.1007/s10531-004-3952-1 (doi:10.1007/s10531-004-3952-1) [DOI] [Google Scholar]
- 19.Curran LM, Caniago I, Paoli GD, Astianti D, Kusneti M, Leighton M, Nirarita CE, Haeruman H. 1999. Impact of El Niño and logging on canopy tree recruitment in Borneo. Science 286, 2184–2188 10.1126/science.286.5447.2184 (doi:10.1126/science.286.5447.2184) [DOI] [PubMed] [Google Scholar]
- 20.Cannon CH, Curran LM, Marshall AJ, Leighton M. 2007. Beyond mast-fruiting events: community asynchrony and individual dormancy dominate woody plant reproductive behavior across seven Bornean forest types. Curr. Sci. 93, 1558–1566 [Google Scholar]
- 21.Cannon CH, Curran LM, Marshall AJ, Leighton M. 2007. Long-term reproductive behaviour of woody plants across seven Bornean forest types in the Gunung Palung national park (Indonesia): suprannual synchrony, temporal productivity and fruiting diversity. Ecol. Lett. 10, 956–969 10.1111/j.1461-0248.2007.01089.x (doi:10.1111/j.1461-0248.2007.01089.x) [DOI] [PubMed] [Google Scholar]
- 22.Lamont BB, Witkowski ETF, Enright NJ. 1993. Postfire litter microsites: safe for seeds, unsafe for seedlings. Ecology 74, 501–512 10.2307/1939311 (doi:10.2307/1939311) [DOI] [Google Scholar]
- 23.Bond WJ, Midgley JJ. 2003. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 164, S103–S114 10.1086/374191 (doi:10.1086/374191) [DOI] [Google Scholar]
- 24.Dietze MC, Clark JS. 2008. Changing the gap dynamics paradigm: vegetative regeneration control on forest response to disturbance. Ecol. Monogr. 78, 331–347 10.1890/07-0271.1 (doi:10.1890/07-0271.1) [DOI] [Google Scholar]
- 25.Cleary DFR, Priadjati A. 2005. Vegetation responses to burning in a rain forest in Borneo. Plant Ecol. 177, 145–163 10.1007/s11258-005-2107-0 (doi:10.1007/s11258-005-2107-0) [DOI] [Google Scholar]
- 26.Kauffman JB. 1991. Survival by sprouting following fire in tropical forests of the eastern Amazon. Biotropica 23, 219–224 10.2307/2388198 (doi:10.2307/2388198) [DOI] [Google Scholar]
- 27.National Institute for Space Research. 2013. Monitoring of the Brazilian Amazon forest by satellite: project PRODES. See http://www.obt.inpe.br/prodes/prodes_1988_2012.htm. (accessed 12 February 2013)
- 28.Aragão L, Shimabukuro YE. 2010. The incidence of fire in Amazonian forests with implications for REDD. Science 328, 1275–1278 10.1126/science.1186925 (doi:10.1126/science.1186925) [DOI] [PubMed] [Google Scholar]
- 29.Soares-Filho BS, et al. 2006. Modelling conservation in the Amazon basin. Nature 440, 520–523 10.1038/nature04389 (doi:10.1038/nature04389) [DOI] [PubMed] [Google Scholar]
- 30.Balch JK, Nepstad DC, Brando PM, Curran LM, Portela O, de Carvalho O, Lefebvre P. 2008. Negative fire feedback in a transitional forest of southeastern Amazonia. Glob. Change Biol. 14, 2276–2287 10.1111/j.1365-2486.2008.01655.x (doi:10.1111/j.1365-2486.2008.01655.x) [DOI] [Google Scholar]
- 31.Oksanen L. 2001. Logic of experiments in ecology: is pseudoreplication a pseudoissue? Oikos 94, 27–38 10.1034/j.1600-0706.2001.11311.x (doi:10.1034/j.1600-0706.2001.11311.x) [DOI] [Google Scholar]
- 32.van Mantgem P, Schwartz M, Keifer MB. 2001. Monitoring fire effects for managed burns and wildfires: coming to terms with pseudoreplication. Nat. Areas J. 21, 266–273 [Google Scholar]
- 33.Hurlbert SH. 1984. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54, 187–211 10.2307/1942661 (doi:10.2307/1942661) [DOI] [Google Scholar]
- 34.Sheil D, May RM. 1996. Mortality and recruitment rate evaluations in heterogeneous tropical forests. J. Ecol. 84, 91–100 10.2307/2261703 (doi:10.2307/2261703) [DOI] [Google Scholar]
- 35.Hurlbert SH. 1971. Nonconcept of species diversity: critique and alternative parameters. Ecology 52, 577–586 10.2307/1934145 (doi:10.2307/1934145) [DOI] [PubMed] [Google Scholar]
- 36.Gotelli NJ, Colwell RK. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391 10.1046/j.1461-0248.2001.00230.x (doi:10.1046/j.1461-0248.2001.00230.x) [DOI] [Google Scholar]
- 37.Oksanen J, et al. 2011. Vegan: Community Ecology Package. R package version 2.0-2. http://CRAN.R-project.org/package=vegan .
- 38.R Development Core Team 2007. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 39.Shipley B. 2000. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Rad JE, Manthey M, Mataji A. 2009. Comparison of plant species diversity with different plant communities in deciduous forests. Int. J. Environ. Sci. Tech. 6, 389–394 [Google Scholar]
- 41.SAS Institute. 2003. SAS System for Windows. Version 9.1.3. Cary, NC: SAS Institute.
- 42.Colwell RK. 2009. EstimateS: Statistical estimation of species richness and shared species from samples. Version 8.2.0. User's Guide and application published at http://purl.oclc.org/estimates. [Google Scholar]
- 43.Chao A, Jost L, Chiang SC, Jiang YH, Chazdon RL. 2008. A two-stage probabilistic approach to multiple-community similarity indices. Biometrics 64, 1178–1186 10.1111/j.1541-0420.2008.01010.x (doi:10.1111/j.1541-0420.2008.01010.x) [DOI] [PubMed] [Google Scholar]
- 44.Chao A, Shen TJ. 2010. Program SPADE (species prediction and diversity estimation). See http://chao.stat.nthu.edu.tw [Google Scholar]
- 45.Tipper JC. 1979. Rarefaction and rarefiction: use and abuse of a method in paleoecology. Paleobiology 5, 423–434 [Google Scholar]
- 46.Ratter JA, Bridgewater S, Ribeiro JF. 2003. Analysis of the floristic composition of the Brazilian cerrado vegetation III: Comparison of the woody vegetation of 376 areas. Edinburgh J. Bot. 60, 57–109 [Google Scholar]
- 47.Silvério DV, Brando PM, Balch JK, Putz FE, Nepstad DC, Oliveira-Santos C, Bustamante MMC. 2013. Testing the Amazon savannization hypothesis: fire effects on invasion of a neotropical forest by native cerrado and exotic pasture grasses. Phil. Trans. R. Soc. B 368, 20120427. 10.1098/rstb.2012.0427 (doi:10.1098/rstb.2012.0427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha S, Howe HF. 2003. Species composition and fire in a dry deciduous forest. Ecology 84, 3118–3123 10.1890/02-3051 (doi:10.1890/02-3051) [DOI] [Google Scholar]
- 49.Ratter JA, Askew GP, Montgomery RF, Gifford DR. 1978. Observations on vegetation of northeastern Mato Grosso. 2. Forests and soils of Rio Suia Missu area . Proc. R. Soc. Lond. B 203, 191–208 10.1098/rspb.1978.0100 (doi:10.1098/rspb.1978.0100) [DOI] [PubMed] [Google Scholar]
- 50.Pool A. 2008. A review of the genus Pyrostegia (Bignoniaceae). Ann. Mont. Bot. Gard. 95, 495–510 10.3417/2003090 (doi:10.3417/2003090) [DOI] [Google Scholar]
- 51.Hoffmann WA, Adasme R, Haridasan M, de Carvalho MT, Geiger EL, Pereira MAB, Gotsch SG, Franco AC. 2009. Tree topkill, not mortality, governs the dynamics of savanna-forest boundaries under frequent fire in central Brazil. Ecology 90, 1326–1337 10.1890/08-0741.1 (doi:10.1890/08-0741.1) [DOI] [PubMed] [Google Scholar]
- 52.Paciorek CJ, Condit R, Hubbell SP, Foster RB. 2000. The demographics of resprouting in tree and shrub species of a moist tropical forest. J. Ecol. 88, 765–777 10.1046/j.1365-2745.2000.00494.x (doi:10.1046/j.1365-2745.2000.00494.x) [DOI] [Google Scholar]
- 53.Bond WJ, Midgley JJ. 2001. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol. 16, 45–51 10.1016/S0169-5347(00)02033-4 (doi:10.1016/S0169-5347(00)02033-4) [DOI] [PubMed] [Google Scholar]
- 54.Veldman JW, Mostacedo B, Pena-Claros M, Putz FE. 2009. Selective logging and fire as drivers of alien grass invasion in a Bolivian tropical dry forest. Forest Ecol. Manag. 258, 1643–1649 10.1016/j.foreco.2009.07.024 (doi:10.1016/j.foreco.2009.07.024) [DOI] [Google Scholar]
- 55.Balch JK, Nepstad DC, Curran LM. 2009. Pattern and process: fire-initiated grass invasion at Amazon transitional forest edges. In Tropical fire ecology: climate change, land use and ecosystem dynamics (ed. Cochrane M.), pp. 481–502 Heidelberg, Germany: Springer-Praxis [Google Scholar]