Abstract
Changes in climate and land use that interact synergistically to increase fire frequencies and intensities in tropical regions are predicted to drive forests to new grass-dominated stable states. To reveal the mechanisms for such a transition, we established 50 ha plots in a transitional forest in the southwestern Brazilian Amazon to different fire treatments (unburned, burned annually (B1yr) or at 3-year intervals (B3yr)). Over an 8-year period since the commencement of these treatments, we documented: (i) the annual rate of pasture and native grass invasion in response to increasing fire frequency; (ii) the establishment of Brachiaria decumbens (an African C4 grass) as a function of decreasing canopy cover and (iii) the effects of grass fine fuel on fire intensity. Grasses invaded approximately 200 m from the edge into the interiors of burned plots (B1yr: 4.31 ha; B3yr: 4.96 ha) but invaded less than 10 m into the unburned plot (0.33 ha). The probability of B. decumbens establishment increased with seed availability and decreased with leaf area index. Fine fuel loads along the forest edge were more than three times higher in grass-dominated areas, which resulted in especially intense fires. Our results indicate that synergies between fires and invasive C4 grasses jeopardize the future of tropical forests.
Keywords: Amazon, tropical, grass invasion, grass–fire cycle, non-native grasses, savannah–forest boundaries
1. Introduction
In many parts of the world, tropical forest–savannah boundaries shift in response to changing climate and disturbance regimes [1,2]. In the southern Amazon Basin, forest is predicted to retreat owing to climate change [3,4] and land-use practices [5,6], which may also facilitate grass invasion and increase both the frequencies and intensities of wildfires. Although some dynamic global vegetation models predict a late-century Amazonian forest dieback [7,8], an integrated view of how this process will occur is still lacking. One potent driver could be the increased forest edge invasion of exotic grasses that accompanies pasture expansion [5]. In addition, fires reportedly interact with grass invasion through a positive feedback cycle, which causes a decline in tree cover, facilitates grass invasion and increases the likelihood of future fires [9–11].
Grass–fire cycles are important on many forest frontiers owing to the combination of increased ignitions, drier forest edges and proliferation of flammable species. For example, several studies document that establishment of invasive grasses, which benefits from reduced tree cover [6,12], increases fine fuel loads, fire intensities [13,14] and grass expansion [6,12–14]. Moreover, frequently burned forests lose carbon storage capacity and may remain in a degraded, low-carbon forest state in which they are susceptible to recurrent fires [15].
There is a wealth of knowledge about forest–savannah boundary dynamics around the world and about how introduced grasses alter vegetation and help create new fire cycles [1,12,13,16]. However, the mechanisms by which grasses expand at the expense of forests are less well understood (but see [11]). In the southeastern Amazon, agricultural expansion [17], selective logging [18] and other land uses may accelerate the rate of reduction of forest cover and enhance the fire–grass cycle by: (i) reducing tree cover and exposing vulnerable forest edges [10,19–21]; (ii) introducing propagules of exotic pasture grasses [11,22]; and (iii) increasing ignitions associated with land management practices [18,23].
Although the importance of fire–grass feedbacks for tropical forests and savannahs is well established [6,9–14], little is known about the mechanisms by which grasses invade forests. In particular, the species composition, extent and fire-related traits that facilitate this process along the substantial amount of degraded forest edges created annually by deforestation need to be documented. In this study, we experimentally evaluated how two fire frequencies (an annual and triennial burn) and the resulting differences in canopy cover interact with grass invasion to change fire behaviour. Specifically, we tested the predictions that: (i) native cerrado (Brazilian savannah) and exotic pasture grass invasion of forests from the edge increases with increases in fire frequency and associated increases in light availability; (ii) the establishment of the exotic grass Brachiaria decumbens from seeds experimentally sown in the forest interior (250 m from forest edges) increases with canopy openness; and (iii) the presence of exotic grasses increases forest fire intensity.
2. Material and methods
(a). Study area
In 2004, we established a large-scale fire experiment in a transitional forest located between the cerrado and Amazon forest biomes (figure 1). This forest showed no signs of previous disturbance by fire or logging and was located in a privately owned ranch in Mato Grosso (13°04′ S, 52°23′ W). The soils in this region are dystrophic red-yellow Oxisols that are deep and well drained [24]. The average annual precipitation of 1700 mm falls predominantly during the October–May rainy season. This forest is less diverse than most wet and moist forests, with only 97 woody species ha−1 greater than 10 cm diameter at breast height [24]; nine of these species represent 56 per cent of the stems [19].
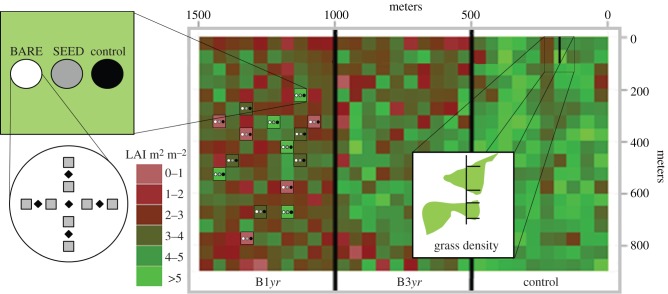
Figure 1.
Experimental design for evaluating B. decumbens establishment in forest interiors and grass density along forest edges. Black diamonds, measure of LAI; grey squares, sample of grass. (Online version in colour.)
(b). Fire experiment
Grass invasion was monitored in a large-scale fire experiment consisting of three 50 ha adjacent plots: one burned annually from 2004 to 2010 (except in 2008; B1yr); one burned every 3 years (2004, 2007 and 2010; B3yr) and an unburned control (figure 1). These large plot sizes allowed us to simulate realistic fires, but did not allow for replication. To deal with this limitation, we conducted pre-fire measurements and established a control plot, following protocols for large-scale unreplicated experiments [25,26]. The experimental burns were all conducted at the end of the dry season (August–September).
The plots bordered pastures until 2006 and soya bean fields afterwards. The dominant grasses in these pastures were B. decumbens Stapf and Andropogon gayanus Kunth, both of which are native to Africa. African grasses were not present in the forest interior before the experimental fires. However, cerrado native grasses (mainly Aristida longifolia; electronic supplementary material, S1) were present along the forest edges prior to fires (less than 5 m), as observed in the control (figure 2). For more details about the fire experiment, see Balch et al. [10,19,24] and Brando et al. [27].
Figure 2.
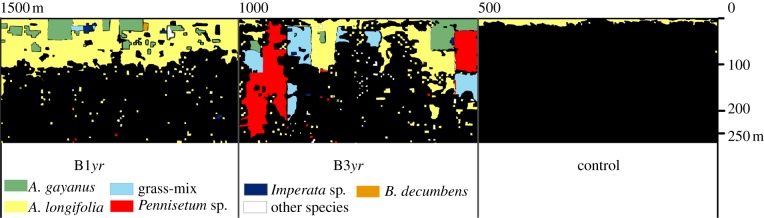
Map of grass invasions from 2004 to 2012 in plots subjected to annual (B1yr) or triennial burns (B3yr) and in the unburned control: Aristida longifolia, Andropogon gayanus, Pennisetum sp., B. decumbens, Imperata sp., grass-mix (area with more than one grass species) and other species (species listed in the electronic supplementary material, S1) (areas in black were not invaded by grasses). (Online version in colour.)
(c). General approach for grass invasion monitoring
To evaluate the determinants and rates of grass invasion in our fire-disturbed experiment, we made two sets of measurements. We first measured grass invasion from the edge into the forest interior annually from 2004 to 2011 in the control, B1yr and B3yr. In 2012, we complemented this measurement by mapping the presence of grasses within 250 m of the forest edge at a resolution of 5 × 5 m (referred to as forest-edge grass invasion) for the control and fire treatment plots. Second, in 2010, we established thirty 100 m long transects (10 per 50 ha plot treatment) along which we estimated the percentage of grass cover and the leaf area index (LAI) of the overstorey (referred to as grass presence). More details about these two set of measurements follow below.
(i). Forest-edge grass invasion
We measured the rate of grass invasion from the forest edge into the interior of each plot 11–12 months following each experimental burn conducted between 2005 and 2011. We mapped the furthest penetration of grass populations along edge-to-interior transects placed every 4 m (n = 125 per plot; details in Balch et al. [10]).To estimate the area invaded by each species, in July 2012, we mapped all areas with greater than 50 per cent grass cover that occurred within 250 m of the nearest forest edge (see the electronic supplementary material, S1; figure 2).
(ii). Grass presence
We estimated percentage grass cover along 30 transects of 100 m in 2010 (10 in each experimental plots) running from the plot edge bordering the field into the forest interior. For each transect, we mapped percentage grass cover (as illustrated in top-right in figure 1) and LAI. Because we measured LAI at discrete points along the transects (0, 10, 30, 50 and 100 m away from edge), we interpolated the LAI using the kriging method described in Pebesma [28] to relate LAI to grass cover.
(d). Experimental manipulation of Brachiaria decumbens populations
To evaluate some factors affecting the establishment of B. decumbens, we conducted a seed-sowing experiment in the forest interior (greater than 250 m from the forest edge), where no pasture grasses were previously present. First, in 2008, we measured LAI in B1yr over 600 points using a LiCor 2000 [29]. Second, we divided the experimental area into three LAI classes: low (less than 2.0); medium (2.0–4.0); and high (greater than 4.0). Third, within every LAI class, we established five blocks comprising three treatments each: a control with no seed inputs; a treatment in which seeds were sown (SEED); and another treatment in which seeds were sown and buried by a rake after the litter layer was removed to increase seed contact with the mineral soil (BARE). Thus, the experiment consisted of 45 circular plots of 50 m2 each, with treatments randomly allocated within LAI classes (figure 1). About 150 viable seeds per square metre of B. decumbens were sown during the rainy seasons of November 2008 and December 2010. We evaluated grass presence and grass cover 6 months after the seeds were sown, and then 2 years after the last planting (February 2012).
(e). Grass effects on fire behaviour
At the end of the 2010 dry season, we classified forest edges on the basis of the dominant vegetation: (i) andropogon (A. gayanus), an African C4 grass commonly planted in pastures; (ii) cuandu, a native C3 grass (A. longifolia), common in Brazilian savannahs [30]; and (iii) mixed vegetation cover, where lianas and sprouts of different woody species were dominant. Within each one of these vegetation types, and in the forest interior (greater than 250 m from forest edges), we quantified the following proxies for forest flammability 5–10 min prior to conducting the experimental fires: (i) fine fuel height (n = 20) using a graduated scale; (ii) fine fuel biomass in 1-m2 plots (n = 6–70); and (iii) fine fuel moisture content (n = 6–100). At the same time, we also measured air temperature and relative humidity using a psychrometer (n = 7–20), and wind direction and speed using a manual anemometer. Finally, we measured flame heights (m) and fire spread rates (m min−1; FSR) on forest edges and in their interiors.
(f). Statistical analysis
We used logistic models to assess the probability of grass invasion in the Grass presence and in Establishment of B. decumbens experiments. First, we developed a model of grass presence as a function of LAI and distance from the edge (model I). Then, we estimated the probability of B. decumbens presence as a function of LAI, seed treatments, proportion of exposed mineral soil and litter thickness (model II). The best models were assumed to be those with the lowest Akaike information criterion [31]. In the text, we present means followed by standard deviations (s.d.) or, in the case of right-skewed distributions, medians followed by the 95% bootstrap confidence intervals (CI).
3. Results
(a). Grass invasion into the forest
The rate of grass invasion was low during the first years of the fire experiment but then increased substantially, particularly after the high-intensity fires of 2007 (see figure 3 and electronic supplementary material, S2). During the 3 years following the initial fires in 2004, for example, grasses invaded only short distances in both fire treatments (1.6–1.9 m ± 0.25–3.69 (± 1 s.d.) yr−1) and in the control (0.3 m ± 0.20 (± 1 s.d.) yr−1). Between 2008 and 2011, by contrast, the rate of grass invasion increased substantially in the burned plots to 13.0 m ± 10.94 (±1 s.d.) yr−1 in B1yr and 19.9 m ± 37.76 (±1 s.d.) yr−1 in B3yr. Although there was a fourfold increase in grass invasion of the control plot, the rate was much lower than in the burned plots (1.2 m ± 3.74).
Figure 3.
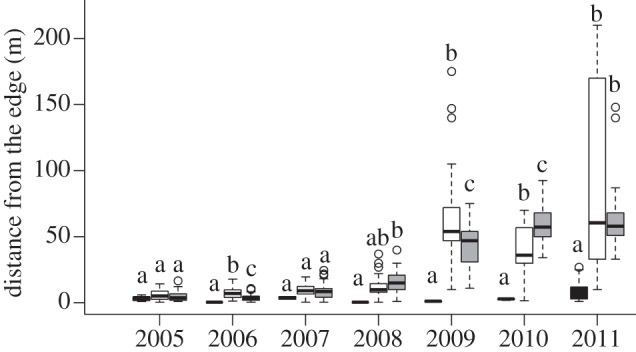
Grass invasion from edges into forest interiors in plots subjected to annual (B1yr) and triennial burns (B3yr) and in an unburned control. Box plots with same letter are not different at p < 0.05 based on Mood's median test [32]. Black boxes, control; white boxes, B3yr; grey boxes, B1yr.
The effects of fire frequency on grass invasion changed over time in the burned plots. In general, grass invasion rates were similar between plots for the period 2005–2007. In the year following the 2007 fires, by contrast, grasses advanced faster in B1yr than in B3yr. In 2010, the rates of grass invasion were 37 per cent lower in B3yr than in B1yr. By contrast, the rates of grass invasion were higher in B3yr than in B1yr in 2009 (13%) and 2011 (4%) (see figure 3 and electronic supplementary material, S2).
By 2012, the total area invaded by grasses was 4.31 ha in B1yr, 4.96 ha in B3yr and 0.33 ha in the control (mostly less than 10 m from the edge; figure 2). The main grass species in B3yr were A. longifolia (38%), Pennisetum sp. (30%) and A. gayanus (10%). In B1yr, these same species were present along the forest edge, but the species dominance differed from B3yr: A. longifolia (82%) and A. gayanus (15%), while in control, only A. longifolia was present (see figure 2 and electronic supplementary material, S1).
(b). Grass presence
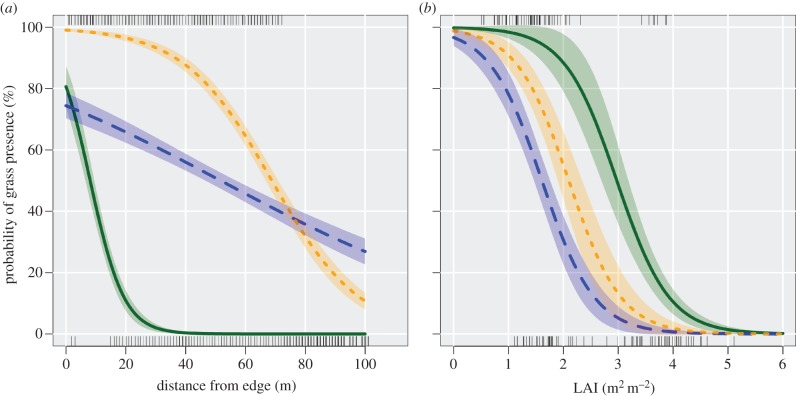
To evaluate the probability of grass invasion into forest interiors, we developed logistic models of grass presence/absence as function of distance from edges and LAI. From these models, we learned that the probability of finding grasses in the forest in 2010 was higher close to edges (model I; figure 4a) and where LAI was low (figure 4b). For example, within 5 m of the forest edges of the burned plots the probability of finding grasses was 72–98%, whereas at 100 m this probability decreased to 11–26%; probabilities were much higher in burned plots than in the control plot (figure 4a). In general, the probability of finding grass in the control plot was 50 per cent or less (i.e. inflection point) at 8.0 m or more from the edge. In B1yr and B3yr, the analogous inflection points were 68.8 m or more and 51.6 m or more, respectively (figure 4a).
Figure 4.
Probability of grass (native and exotic) (a) presence as function of distance from the edge and (b) LAI up to 100 m from the edge in annual (B1yr) and triennial burns (B3yr) and in an unburned control. Solid lines, control; dashed lines, B3yr; dotted lines, B1yr. (Online version in colour.)
The probability of grass invasion decreased as a function of increasing LAI but at rates that differed among treatments. While the intercepts of our logistic model were similar between the burned plots and the control, the slopes were not (see figure 4b and electronic supplementary material, S3). As a result, the inflection points of this model differed among treatments, being higher in the control (3.0 m² m−2), medium in B1yr (2.1 m² m−2) and lowest in B3yr (1.6 m² m−2). These results indicate that the differences in the probability of grass invasion between treatments were not fully explained by LAI, although our full logistic model explained 60 per cent of the variability in the data (pseudo r² = 0.60).
(c). Establishment of Brachiaria decumbens
Based on the logistic models derived from model II, we found that the presence of B. decumbens was strongly influenced by LAI and seed additions (see figure 5 and electronic supplementary material, S5), but not by the fraction of mineral soil exposed or litter thickness (see the electronic supplementary material, S4). For example, where seeds of B. decumbens were sown, the probability of finding any grass 6 months later was 50 per cent or higher when LAI was less than or equal to 2.45–3.14. In the control (without seed addition), the presence of B. decumbens was low for all levels of LAI (figure 5).
Figure 5.
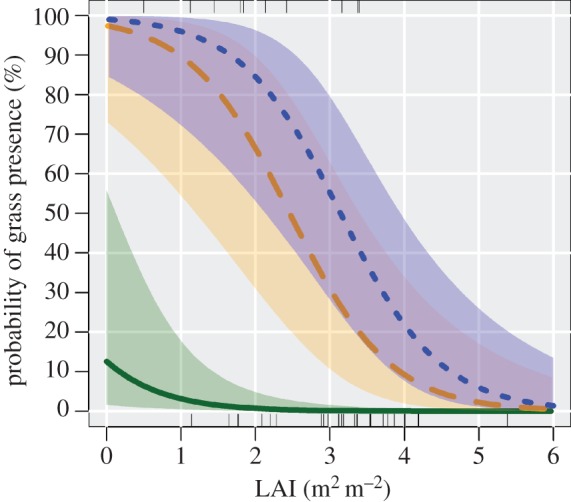
Predicted B. decumbens establishment as a function of LAI for two treatments with seed sowing and the control. Solid line, control; dashed line, SEED; dotted line, BARE. (Online version in colour.)
While B. decumbens occurred where canopy cover was sparse, it covered only small portions of the experimental plots. In the SEED treatment, for example, B. decumbens covered 1.0 ± 0.22% (s.e.) of the circular plots in 2010, although it increased to 10.0 ± 2.30% (s.e.) in 2012. In the BARE treatment, where the seeds were sown after the soil surface was raked, even 2 years after seeding the ground covered by B. decumbens was only 4.4 ± 1.14% (see the electronic supplementary material, S6). Because its ground cover was less than 10 per cent, the establishment of this grass is expected to have little current effect on fuel loads.
(d). Grass invasion and fire behaviour
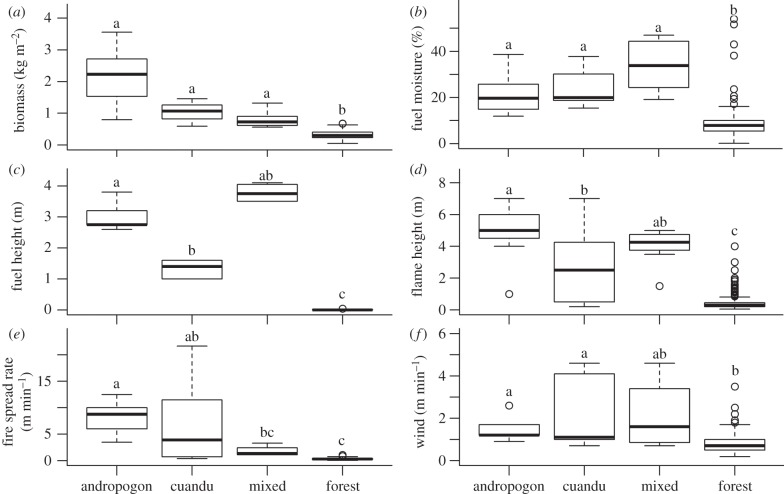
In August 2010, fine fuel loads along the forest edge were highest in areas dominated by exotic grasses (2.1 ± 2.2 kg m−2 (s.d.)), medium in areas dominated by native grass (1.0 ± 0.34 kg m−2 (s.d.)) and lowest in mixed vegetation types (0.8 ± 0.28 kg m−2 (s.d.)). In the forest interior, fine fuel loads (comprising mostly leaves and small twigs) were lower (0.3 ± 0.13 kg m−2 (s.d.); figure 6a).
Figure 6.
(a) Biomass, (b) moisture, (c) standing height, (d) flame height, (e) fire spread rate and (f) wind speed in areas with a predominance of A. gayanus (andropogon), A. longifolia (cuandu), mixed (environments dominated by lianas and woody sprouts) and forest fuels. Box plots with same letter are not different at p < 0.05 based on Mood's median test [32].
Fire intensity (as measured during the fires of 2010) generally increased with fine fuel loads (figure 6d,e). Similarly, FSR were highest in andropogon (8.7 m−1 min−1; CI: 5.0–13.4), medium in cuandu (3.90 m−1 min−1; CI: 5.0–10.4) and mixed vegetation types along the forest edge (1.35 m−1 min−1; CI 0.0–2.4) and lowest in the forest interior (0.29 m−1 min−1; CI: 0.25–0.32; figure 6f). When we used flame height as a proxy for fire intensity, we observed the same pattern with one exception. Flame heights were higher than expected in the mixed vegetation type (figure 6d), which suggests that we did not adequately measure the fuel loads.
4. Discussion
(a). Fire-induced loss of canopy cover encourages grass invasion, where seed is available
Results from our large-scale manipulative experiment corroborate the hypothesis that by killing trees, understorey fires promote grass establishment in neotropical forests, with important effects on local fire regimes and the likelihood of ecosystem transition. As fire-induced tree mortality thinned the canopy [27,33] and allowed more radiation to reach the forest floor, grasses advanced rapidly from the forest edge, where seed availability was not a constraint for invasion. Because these grasses accumulated more fine fuel above-ground than the C3 woody vegetation they replaced [34], fires were more intense where grasses were more abundant, especially along forest edges. This is one of the first studies in the Amazon that experimentally documents the frequently posited abrupt transition from tree to grass dominance, with evidence for a strong positive feedback between grass fuels and fire intensity [9].
Whereas, canopy cover declined substantially across most of the burned plots [33], grasses mostly occurred less than 200 m from forest edges. This result points to dispersal limitations on the rate of grass invasion over the 8 years of the experiment. For exotic C4 grasses, this spatial pattern was expected given that pastures bordered the experimental plots for more than 20 years. Apparently, while wind, animals, insects and human visitors dispersed some grass seeds into the forest interior [35], dispersal was limited. Also noteworthy is the presence of native cerrado species in the area although cerrado patches begin more than 50 km from the experimental forest [17].
The spatial–temporal pattern of grass invasion was influenced not only by canopy density and seed availability, but also by the physiological characteristics of the grasses growing along the forest edge. Aristida longifolia was the only grass species to invade the control plot, probably because this a native C3 species with lower light requirements than most C4 pasture grasses [30,36]. This shade tolerance probably enables A. longifolia to invade forests even when canopy cover is relatively dense, which may explain why it was the most common invader of the experimental plots following the low-intensity fires of 2004. Once established, however, this grass species tended to accumulate enough above-ground fine fuel to promote higher intensity, tree-killing fires, which opened the canopy and facilitated the invasion of exotic, light-demanding C4 pasture grasses. Because these C4 grasses accumulated more fuel than A. longifolia or the native woody vegetation, fires became more intense over time along the forest edge (figure 6). This pattern was observed in B3yr, but not in B1yr, where A. longifolia remained the most dominant species over the duration of the experiment.
(b). Fire selects for native and exotic grasses
Results from our seeding experiment with B. decumbens reinforce the notion that variables other than light availability (e.g. herbivory, pathogens and competition) constrain the establishment of exotic grasses in forest interiors [35,37]. For example, while we sowed seeds of B. decumbens in quantities considered high enough to overcome seed limitation (150 kg ha−1) [38], this African pasture grass covered only a small fraction of the experimental subplots. Low light levels could explain this invasion failure, but LAI ranged widely across the experimental subplots and some areas were open (LAI < 0.5 m2 m−2). High seed predation could also explain the low capacity of B. decumbens to invade our fire-disturbed forest, but we found no effect of the BARE treatments in which seeds were buried to reduce predation and promote germination. Hence, it is still unclear why so few B. decumbens seeds germinated and why there was no substantial grass expansion across the heavily seeded subplots, but herbivory, pathogens and above- and below-ground competition probably contributed to this pattern [35,37]. These findings notwithstanding, the 10-fold increase in grass expansion in the SEED treatment from 2010 to 2012 (see the electronic supplementary material, S6) suggests that populations of this grass species will expand further over time.
(c). Persistence of fire-promoting grasses
While grass establishment transformed large portions of the forest edges into savannah-like ecosystems, it is still unclear whether this novel ecosystem will persist. Two years after the most recent fire, grasses still covered approximately 32–37% of the experimental forest edge, suggesting that this grassy ecosystem is at least semi-permanent. But over longer, fire-free time periods (e.g. decades), remnant tree growth and tree regeneration from resprouts and seedlings are expected to outcompete grasses. For instance, in eastern Amazonian moist forests, abandoned pastures give rise to secondary forests in 4–20 fire-free years, depending on the pasture-use history [39,40]. Similarly, Uhl et al. [41] found that a forest subjected to a slash-and-burn agriculture began shading grasses within a year of abandonment. Finally, Kauffman [42] found that in a burned and logged forest in Paragominas (Pará), 75 per cent of tree species had the capacity to resprout after fire in a logged forest and that the mean resprout height reached 4.2 m 20 months after the disturbance. Based on these studies and others [39,43], it seems likely that fire-disturbed forests can recover over years or decades even in the presence of grasses, at least if there is natural regeneration of woody plants and fires cease. In our study site, the grass invasion front retreated over time since fire in the burned plots, supporting this proposition.
After episodes of intense and frequent fires that exceed forest resilience, recovery may require many fire-free decades. Given that fires are increasing in the study area, it seems more likely that many fire-disturbed forests will permanently transition into grassy ecosystems. Moreover, the greater species evenness at intermediate disturbances (burn every three years) perhaps suggests different fire frequencies will select for a suite of different grass species at degraded forest edges [1,5,21,44]. Repeated and severe fires not only alter forest structure and microclimate in ways that facilitate grass invasion and establishment, but also reduce the competitiveness of the native woody vegetation; fires (i) kill seed trees [19], (ii) increase seed predation of native tree species [45], (iii) lower seed germination [10,44,46] and (iv) reduce tree seedling density and diversity [46,47]. Despite these insights, the question of how much disturbance is too much to cause a change in the state of this system is still hard to answer. Empirical evidence suggests that instead of grass domination, fire-degraded forests may become dominated by vines, resprouts and pioneer tree species [15,23,41], while the presence of grasses is expected to be transient and strongly associated with high fire frequencies.
(d). Implications
Major increases in grass invasion occurred following intense fires associated with drought events. This result suggests that fire could mediate the predicted climate-driven substitution of large portions of Amazonian forest by grass-dominated ecosystems. With the increases in air temperatures and decreases in precipitation predicted for the Amazon [4,48,49], fires are expected to become more frequent and intense [5,21,23,44], killing proportionally more trees. Although seed dispersal may limit invasion into fire-disturbed forests, seeds of exotic grasses are already abundant [5,17], particularly in the Xingu Basin where pastures cover much of the landscape [17]. While seeds of exotic C4 grasses are abundant only along forest edges, approximately 16 per cent of Xingu Basin forests are less than 200 from a forest-pasture edge [33].
Although understorey fires and grass invasion can tip the balance between grass and tree domination, this process is not well represented in most dynamic vegetation models. In general, these models assume that there is no seed limitation on grass establishment [50]. For those models that do represent fire disturbance, fire behaviour is not differentiated between forests and grass-dominated environments. Our results show the need to incorporate fire in these models and to differentiate between forest understorey fires with and without the presence of C4 African grasses.
Acknowledgements
This study was supported by the Gordon and Betty Moore Foundation, Packard Foundation and the National Science Foundation (grant no. 0743703). We thank L. Curran, M. Coe, E. Davidson and O. Carvalho for help with the experimental design and the crew from IPAM for their contribution with data collection (in particular S. Rocha and D. Nunes), and we also thank Dra. Regina C. Oliveira for the grasses species identification. CAPES provided support for the first author's dissertation research. We are thankful for the helpful comments from two anonymous reviewers.
References
- 1.Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232 10.1126/science.1210465 (doi:10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 2.Sankaran M, et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849 10.1038/nature04070 (doi:10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 3.Hutyra LR, Munger JW, Nobre CA, Saleska SR, Vieira SA, Wofsy SC. 2005. Climatic variability and vegetation vulnerability in Amazônia. Geophys. Res. Lett. 32, 2–5 10.1029/2005GL024981 (doi:10.1029/2005GL024981) [DOI] [Google Scholar]
- 4.Coe MT, et al. 2013. Deforestation and climate feedbacks threaten the ecological integrity of south–southeastern Amazonia. Phil. Trans. R. Soc. B 368, 20120155. 10.1098/rstb.2012.0155 (doi:10.1098/rstb.2012.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nepstad DC, Stickler CM, Soares-filho B, Merry F, Nin E. 2008. Interactions among Amazon land use, forests and climate: prospects for a near-term forest tipping point. Phil. Trans. R. Soc. B 363, 1737–1746 10.1098/rstb.2007.0036 (doi:10.1098/rstb.2007.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldman JW, Mostacedo B, Peña-Claros M, Putz FE. 2009. Selective logging and fire as drivers of alien grass invasion in a Bolivian tropical dry forest. Forest Ecol. Manage. 258, 1643–1649 10.1016/j.foreco.2009.07.024 (doi:10.1016/j.foreco.2009.07.024) [DOI] [Google Scholar]
- 7.Cox PM, Betts RA, Collins M, Harris PP, Huntingford C, Jones CD. 2004. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theor. Appl. Climatol. 78, 157–175 10.1007/s00704-004-0050-y (doi:10.1007/s00704-004-0050-y) [DOI] [Google Scholar]
- 8.Huntingford C, Harris PP, Gedney N, Cox PM, Betts RA, Marengo JA. 2004. Using a GCM analogue model to investigate the potential for Amazonian forest dieback. Theor. Appl. Climatol. 78, 177–185 10.1007/s00704-004-0051-x (doi:10.1007/s00704-004-0051-x) [DOI] [Google Scholar]
- 9.D'Antonio CM, Vitousek PM. 1992. Biological invasions by exotic grasses, the grass–fire cycle, and global change. Annu. Rev. Ecol. Syst. 23, 63–87 10.1146/annurev.es.23.110192.000431 (doi:10.1146/annurev.es.23.110192.000431) [DOI] [Google Scholar]
- 10.Balch JK, Nepstad DC, Curran LM. 2009. Pattern and process: fire-initiated grass invasion at Amazon transitional forest edges. In Tropical fire ecology (ed. Cochrane M.), pp. 481–502 Chichester, UK: Springer [Google Scholar]
- 11.Veldman JW, Putz FE. 2011. Grass-dominated vegetation, not species-diverse natural savanna, replaces degraded tropical forests on the southern edge of the Amazon Basin. Biol. Conserv. 144, 1419–1429 10.1016/j.biocon.2011.01.011 (doi:10.1016/j.biocon.2011.01.011) [DOI] [Google Scholar]
- 12.Hoffmann WA, Lucatelli VMPC, Silva FJ, Azeuedo INC, Marinho MDS, Albuquerque AMS, Lopes ADO, Moreira SP. 2004. Impact of the invasive alien grass Melinis minutiflora at the savanna-forest ecotone in the Brazilian Cerrado. Divers. Distrib. 10, 99–103 10.1111/j.1366-9516.2004.00063.x (doi:10.1111/j.1366-9516.2004.00063.x) [DOI] [Google Scholar]
- 13.Setterfield SA, Rossiter-Rachor NA, Hutley LB, Douglas MM, Williams RJ. 2010. Biodiversity research: turning up the heat: the impacts of Andropogon gayanus (gamba grass) invasion on fire behaviour in northern Australian savannas. Divers. Distrib. 16, 854–861 10.1111/j.1472-4642.2010.00688.x (doi:10.1111/j.1472-4642.2010.00688.x) [DOI] [Google Scholar]
- 14.Rossiter NA, Setterfield SA, Douglas MM, Hutley LB. 2003. Testing the grass–fire cycle: alien grass invasion in the tropical savannas of northern Australia. Divers. Distrib. 9, 169–176 10.1046/j.1472-4642.2003.00020.x (doi:10.1046/j.1472-4642.2003.00020.x) [DOI] [Google Scholar]
- 15.Zarin DJ, et al. 2005. Legacy of fire slows carbon accumulation in Amazonian forest regrowth. Front. Ecol. Environ. 3, 365–369 10.1890/1540-9295(2005)003[0365:LOFSCA]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0365:LOFSCA]2.0.CO;2) [DOI] [Google Scholar]
- 16.Hirota M, Holmgren M, Van Nes EH, Scheffer M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235 10.1126/science.1210657 (doi:10.1126/science.1210657) [DOI] [PubMed] [Google Scholar]
- 17.Macedo MN, DeFries RS, Morton DC, Stickler CM, Galford GL, Shimabukuro YE. 2012. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proc. Natl Acad. Sci. USA 109, 1341–1346 10.1073/pnas.1111374109 (doi:10.1073/pnas.1111374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nepstad DC, et al. 1999. Large-scale impoverishment of Amazonian forests by logging and fire. Nature 1405, 505–508 10.1038/19066 (doi:10.1038/19066) [DOI] [Google Scholar]
- 19.Balch JK, Nepstad DC, Curran LM, Brando P, Portela O, Guilherme P, Reuning-Scherer JD, De Carvalho O., Jr 2011. Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. Forest Ecol. Manage. 261, 68–77 10.1016/j.foreco.2010.09.029 (doi:10.1016/j.foreco.2010.09.029) [DOI] [Google Scholar]
- 20.Laurance WF, et al. 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618 10.1046/j.1523-1739.2002.01025.x (doi:10.1046/j.1523-1739.2002.01025.x) [DOI] [Google Scholar]
- 21.Morton DC, Le Page Y, DeFries R, Collatz GJ, Hurtt GC. 2013. Undestorey fire frequency and the fate of burned forests in southern Amazonia. Phil. Trans. R. Soc. B 368, 20120163. 10.1098/rstb.2012.0163 (doi:10.1098/rstb.2012.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldman JW, Putz FE. 2010. Long-distance dispersal of invasive grasses by logging vehicles in a tropical dry forest. Biotropica 42, 697–703 10.1111/j.1744-7429.2010.00647.x (doi:10.1111/j.1744-7429.2010.00647.x) [DOI] [Google Scholar]
- 23.Cochrane M, Alencar A, Schulze M, Souza C, Nepstad DC, Lefebvre P, Davidson EA. 1999. Positive feedbacks in the fire dynamic of closed canopy tropical forests. Science 284, 1832–1835 10.1126/science.284.5421.1832 (doi:10.1126/science.284.5421.1832) [DOI] [PubMed] [Google Scholar]
- 24.Balch JK, Nepstad DC, Brando P, Curran LM, Portella O, de Carvalho JRO, Lefebvre P. 2008. Negative fire feedback in a transitional forest of southeastern Amazonia. Glob. Change Biol. 14, 2276–2287 10.1111/j.1365-2486.2008.01655.x (doi:10.1111/j.1365-2486.2008.01655.x) [DOI] [Google Scholar]
- 25.Rasmussen PW, Heisey DM, Nordheim EV, Frost TM. 2001. Time-series intervention analysis: unreplicated large-scale experiments. In Design and analysis of ecological experiments (eds Scheiner SM, Gurrevitch JG.), pp. 138–158 New York, NY: Chapman & Hall [Google Scholar]
- 26.Carpenter SR. 1998. The need for large-scale experiments to assess and predict the response of ecosystems to perturbation. In Successes limitations and frontiers in ecosystem science (eds Pace ML, Groffman PM.), pp. 287–312 Berlin, Germany: Springer [Google Scholar]
- 27.Brando P, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe MT, Putz FE. 2012. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density, and fire behavior. Glob. Change Biol. 18, 630–641 10.1111/j.1365-2486.2011.02533.x (doi:10.1111/j.1365-2486.2011.02533.x) [DOI] [Google Scholar]
- 28.Pebesma EJ. 2004. Multivariable geostatistics in S: the gstat package. Comput. Geosci. 30, 683–691 10.1016/j.cageo.2004.03.012 (doi:10.1016/j.cageo.2004.03.012) [DOI] [Google Scholar]
- 29.LI-COR 1992. LAI-2000 plant canopy analyzer. Lincoln, NE: LI-COR Inc [Google Scholar]
- 30.Longhi-Warner H. 1999. O gênero Aristida (Poaceae) no Brasil. Bol. Inst. Bot. 12, 113–179 [Google Scholar]
- 31.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer [Google Scholar]
- 32.Sprent P, Smeeton NC. 2001. Applied nonparametric statistical methods, 3rd edn. London, UK: Chapman and Hall [Google Scholar]
- 33.Brando PM, et al. Submitted. Abrupt Amazon forest dieback due to drought-fire interactions. [Google Scholar]
- 34.Ehleringer JR, Monson RK. 2009. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 24, 411–439 10.1146/annurev.es.24.110193.002211 (doi:10.1146/annurev.es.24.110193.002211) [DOI] [Google Scholar]
- 35.Gibson DJ. 2009. Grasses and grassland ecology. New York, NY: Oxford University Press [Google Scholar]
- 36.Cerros-Tlatilpa R, Columbus JT. 2009. C3 photosynthesis in Aristida longifolia: implication for photosynthetic diversification in Aristidoideae (Poaceae). Am. J. Bot. 96, 1379–1387 10.3732/ajb.0800265 (doi:10.3732/ajb.0800265) [DOI] [PubMed] [Google Scholar]
- 37.Valerio JR, Lapointe SL, Kelemu S, Fernandes CD, Morales FJ. 1996. Pests and diseases of Brachiaria species. In Brachiaria: biology, agronomy, and improvement (eds Miles JW, Maas JS, Valle CB.), pp. 86–105 Cali, Colombia: Centro Internacional de Agricultura Tropical [Google Scholar]
- 38.Santos-Filho LE. 1996. Seed production: perspective from the Brasilian private sector. In Brachiaria: biology, agronomy, and improvement (eds Miles JW, Maass JS, Valle CB.), pp. 141–147 Cali, Colombia: Centro Internacional de Agricultura Tropical [Google Scholar]
- 39.Hughes RF, Kauffman JB, Cummings DL. 2000. Fire in the Brazilian Amazon 3. Dynamics of biomass, C, and nutrient pools in regenerating forests. Oecologia 124, 574–588 10.1007/S004420000416 (doi:10.1007/S004420000416) [DOI] [PubMed] [Google Scholar]
- 40.Uhl C, Buschbacher R, Serrao EAS. 1988. Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J. Ecol. 76, 663–681 10.2307/2260566 (doi:10.2307/2260566) [DOI] [Google Scholar]
- 41.Uhl C, Clark H, Clark K, Maquirino P. 1982. Successional patterns associated with slash-and-burn agriculture in the upper Rio Negro region of the Amazon Basin. Biotropica 14, 249–254 10.2307/2388082 (doi:10.2307/2388082) [DOI] [Google Scholar]
- 42.Kauffman JB. 1991. Survival by sprouting following fire in tropical forests of the Eastern Amazon. Biotropica 23, 219–224 10.2307/2388198 (doi:10.2307/2388198) [DOI] [Google Scholar]
- 43.Barlow J, Peres CA. 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Phil. Trans. R. Soc. B 363 1787–1794 10.1098/rstb.2007.0013 (doi:10.1098/rstb.2007.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cochrane M, Laurance WF. 2002. Fire as a large-scale edge effect in Amazonian forests. J. Trop. Ecol. 1, 311–325 10.1017/S0266467402002237 (doi:10.1017/S0266467402002237) [DOI] [Google Scholar]
- 45.Carvalho KS, Balch J, Moutinho P. 2012. Influências de Atta spp (Hymenoptera: Formicidae) na recuperação da vegetação pós fogo em floresta de transição amazônica. Acta Amaz. 42, 81–88 10.1590/S0044-59672012000100010 (doi:10.1590/S0044-59672012000100010) [DOI] [Google Scholar]
- 46.Massad TJ, et al. 2012. Interactions between repeated fire, nutrients, and insect herbivores affect the recovery of diversity in the southern Amazon. Oecologia (doi:10.1007/s00442-012-2482-x) [DOI] [PubMed] [Google Scholar]
- 47.Balch JK, Massad TJ, Brando PM, Nepstad DC, Curran LM. 2013. Effects of high-frequency understorey fires on woody plant regeneration in southeastern Amazonian forests. Phil. Trans. R. Soc. B 368, 20120157. 10.1098/rstb.2012.0157 (doi:10.1098/rstb.2012.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa MH, Foley JA. 1997. Water balance of the Amazon Basin: dependence on vegetation cover and canopy conductance. J. Geophys. Res. 102, 23 973–23 989 10.1029/97JD01865 (doi:10.1029/97JD01865)11541236 [DOI] [Google Scholar]
- 49.Spracklen DV, Arnold SR, Taylor CM. 2012. Observations of increased tropical rainfall preceded by air passage over forests. Nature 489, 282–285 10.1038/nature11390 (doi:10.1038/nature11390) [DOI] [PubMed] [Google Scholar]
- 50.Soares-Filho B, et al. 2012. Forest fragmentation, climate change and understory fire regimes on the Amazonian landscapes of the Xingu headwaters. Landscape Ecol. 27, 585–598 10.1007/s10980-012-9723-6 (doi:10.1007/s10980-012-9723-6) [DOI] [Google Scholar]