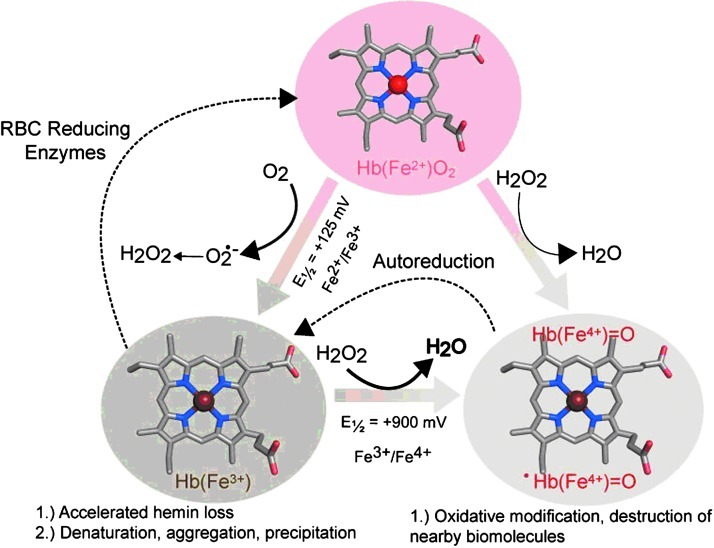
FIG. 1.
Iron-centered oxidative transitions within hemoglobin (Hb). Hemin iron atoms within Hb undergo spontaneous oxidation from ferrous to ferric oxidation states. This process indirectly produces hydrogen peroxide, which can further react with ferric and ferrous Hb to produce ferryl species. Reductases within red blood cells keep the heme iron in the ferrous state. Redox potentials were obtained from Banerjee et al. (3). Figure constructed using the Illustrator (Adobe Systems Incorporated, Mountain View, CA) and PyMOL Molecular Graphics System (Schroödinger, LLC, New York, NY).