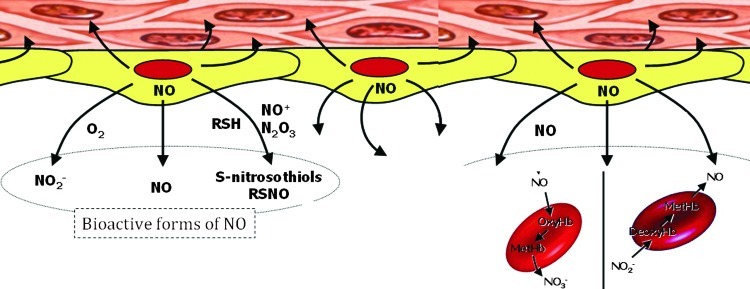
FIG. 3.
Bioactive NO forms. In plasma, NO may react with molecular oxygen to form nitrite (NO2−) or with superoxide (O2−) to form peroxynitrite (OONO−), which subsequently decomposes to yield nitrate (NO3−). Alternatively, the nitrosonium moiety of NO may react with thiols to form nitrosothiols (RSNO). Furthermore, NO may reach the erythrocytes (RBCs) to react with either oxyhemoglobin to form methemoglobin (metHb) and NO2−, with deoxyhemoglobin to form nitrosylhemoglobin (NOHb), or with the Cys93 residue of the β-subunit to form S-nitrosohemoglobin (SNOHb). In addition, plasma NO2− could be taken up by RBCs, where it is oxidized in an Hb-dependent manner to NO3−. Auto-oxidation of NO in an aqueous environment leads to the formation of dinitrogen trioxide (N2O3). This intermediate can nitrosate and oxidize different substrates to yield either nitrosamines or S-nitrosothiol adducts (RSNO). Some well-documented RSNO in plasma are SNOAlb, S-nitrosoalbumin; GSNO, S-nitrosoglutathione; CysNO, S-nitrosocysteine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.