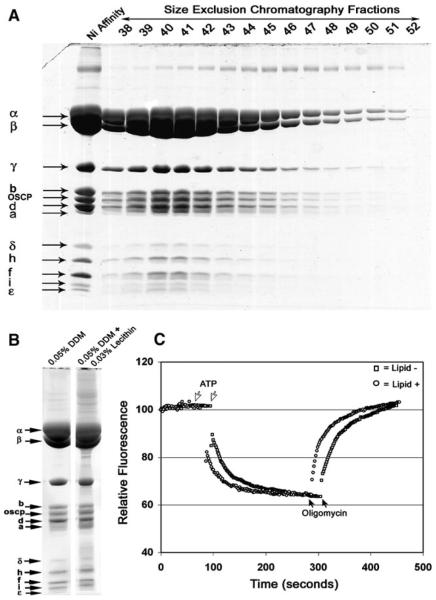
Fig. 1.
Mono-disperse preparation of coupled yeast ATP synthase. a The elution profile as analyzed by SDS PAGE from a Superose 6 size exclusion column in the presence of 0.05% DDM. The first lane shows the peptides from the enzyme purified from the Ni-Sepharose column. The fractions peak of at 42 min (0.3 ml/min) is consistent with a molecular weight of 650,000 Da. b The peptide profile as analyzed by SDS PAGE of the enzyme purified in the absence and presence of 0.03% lecithin. c The enzyme purified in the absence and presence of lecithin were incorporated into liposomes and tested for proton pumping coupled to ATP hydrolysis as described in Materials and Methods. The acidification of the internal liposome space was monitored by the fluorescence quenching of 9ACMA. The error bars show 1 standard error in this and all of the figures